Efficient and convenient preparation of β-nitrostyrene derivatives by using Henry condensation under ecologically benign solvent-free conditions
HU Xiaoyun, WANG Cui, TANG Yanliu, ZHU Wei
(College of Chemistry and Materials, South-Central University for Nationalities, Wuhan 430074, China)
Abstract An effective and convenient preparation method of β-nitrostyrene derivatives by using Henry condensation under mild and green conditions was presented. The essential advantage of this method was solvent-free, which employed environmentally benign functionalized ionic liquid as catalyst instead of conventional applied acids or bases. The method could attain good to excellent conversion of the aromatic aldehydes and high yields of the corresponding β-nitrostyrene derivatives. Furthermore, the ionic liquid could be quantitatively recovered after the reaction.
Keywords Henry condensation; solvent free; functionalized ionic liquid; β-nitrostyrene derivatives
Theβ-nitrostyrene derivatives are widely employed as key intermediates in synthetic organic chemistry[1]. Furthermore, this type of compounds has important medical applications exhibiting anti-cancer[2-3], anti-microbial[4-5], anti-inflammatory[6], and anti-platelet activities[7]. Conventionally, theβ-nitrostyrene derivatives are synthesized in a moderate-to-good yield via Henry condensation catalyzed by acids or bases. The most significant side reactions were found to be trimerisation ofβ-nitrostyrenes into 1,3,5-trisubstituted arenes[8]and polymerization giving intractable structures[9]. Besides, most of these synthetic methods require the use of organic solvents and have some limitations such as a tedious work-up procedure and difficulty of the catalyst recovery. These obvious shortcomings prompt us to develop an efficient method to improve the synthesis ofβ-nitrostyrene derivatives.
Recently, ionic liquids attracted much attention of organic chemists as unique “green” solvents due to their nontoxic and non-volatile nature.Moreover, their good thermal and chemical stability predetermines a high degree of recyclability[10]. Furthermore, functionalized ionic liquids, in which a functional group is covalently tethered to the corresponding cation or anion(or both) of ionic liquid, have been developed not only as a reaction medium but also as a reagent or catalyst in organic synthesis[11]. Since the pioneered work conducted by Davis′s group[12], several successful results of the functionalized ionic liquids application have been reported so far[13-16].Besides the discussed functionalized ionic liquids, 2-hydroxyethyl ammonium acetate is one of the best suited candidates owing to the facile preparation procedure and low cost, which was firstly synthesized as a basic ionic liquid to absorb acidic waste[17]. However, there are only a few reports on its application as a catalyst in synthetic organic chemistry[18]. Also, a series of chalcones by using 2-hydroxyethyl ammonium acetate as functionalized ionic liquid were obtained[19]. Herein this paper reported a highly efficient Henry condensation catalyzed by 2-hydroxyethyl ammonium acetate under solvent-free condition at room temperature for the first time, therefore providing a practical and environmentally friendly method for the synthesis ofβ-nitrostyrene derivatives(Fig.1).
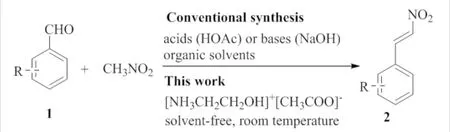
Fig.1 General reaction scheme for the synthesis of β-nitrostyrene derivatives圖1 β-硝基苯乙烯衍生物的一般合成方案
1 Experimental
1.1 Materials and instruments
Nitromethane and various aldehydes were purchased and used as received, 2-hydroxyethyl ammonium acetate was synthesized according to the literature[17]. For the spectral data of products, see Supporting Information.
NMR spectra were performed on a Varian Mercury VS 300 or Bruker Avance III 600; Mp: VEB W?getechnik Rapido PHMK 05, uncorrected.
All the synthesized compounds were properly characterized by their spectroscopic data and Mp. The known compounds are identified by comparison of the corresponding spectroscopic data and melting points with the reported data in the literatures.
1.2 Preparation of 2-hydroxyethyl ammonium
acetate
The ionic liquid was prepared quantitatively and in high purity according to the corresponding literature[17], which is identified by comparison of its spectroscopic data with it reported in the literature.1H NMR(300 MHz, DMSO)δ: 1.69(s, 3H), 2.74(d, 2H,J= 4.5 Hz), 3.49(d, 2H,J= 4.8 Hz), 5.56(broad, 4H, -OH and -NH3).
1.3 General procedure for the preparation of β-nitrostyrene derivatives
In a test tube equipped with a magnetic bar, benzaldehyde(5 mmol), nitromethane(6 mmol), and 2-hydroxyethyl ammonium acetate(0.5 mmol) were charged, the mixture was stirred at certain temperature and time(see Tab.1). Then the reaction was quenched by addition of water(3 mL), the precipitate matter was filtered and recrystallized with 80% ethanol to give (E)-(2-nitrovinyl)benzene(2a)[20], yield 81%; mp 55-56 ℃;1H NMR(300 MHz, CDCl3):δ8.02(d,J=13.5 Hz, 1H ), 7.60(d,J=14.7 Hz, 1H), 7.57-7.44(m,5H).
(E)-1-methyl-4-(2-nitrovinyl)benzene(2b)[20]: yield 92%; mp 105-108 ℃;1H NMR(600 MHz, CDCl3):δ7.98(d,J= 13.6 Hz, 1H), 7.56(d,J= 13.6 Hz, 1H), 7.44(d,J= 8.1 Hz, 2H), 7.25(d,J= 7.9 Hz, 2H), 2.41(s, 3H).
(E)-1-methoxy-4-(2-nitrovinyl)benzene(2c)[21]: yield 86%; mp 78-80 ℃;1H NMR(600 MHz, CDCl3):δ7.97(d,J=13.6 Hz, 1H), 7.51(dd,J= 13.9, 7.6 Hz, 3H), 6.99-6.92(m, 2H), 3.87(s, 3H).
(E)-1-methoxy-3-(2-nitrovinyl)benzene(2d)[20]: yield 92%; mp 78-80 ℃;1H NMR(600 MHz, CDCl3):δ7.96(d,J= 13.6 Hz, 1H), 7.57(d,J= 13.6 Hz, 1H), 7.39-7.34(m, 1H), 7.14(d,J= 7.6 Hz, 1H), 7.08-7.00(m, 2H), 3.85(s, 3H).
(E)-1-(N,N-dimethyl)-4-(2-nitrovinyl)benzene(2e)[20]: yield 87%; mp 182-184 ℃;1H NMR(300 MHz, CDCl3):δ8.00-7.95(d,J= 13.8 Hz, 1H), 7.53-7.48(d,J= 13.2 Hz, 1H), 7.44-7.42(d,J= 7.2 Hz, 2H,), 6.69-6.67(d,J=7.8 Hz, 2H), 3.08(s, 6H, 3H).
(E)-1-chloro-4-(2-nitrovinyl)benzene(2f)[21]: yield 88%; mp 108-110 ℃;1H NMR(600 MHz, CDCl3):δ7.96(d,J= 13.7 Hz, 1H), 7.57(d,J= 13.7 Hz, 1H), 7.49(d,J= 8.4 Hz, 2H), 7.43(d,J= 8.4 Hz, 2H).
(E)-2,4-dichloro-1-(2-nitrovinyl)benzene(2g)[20]:yield 85%; mp 112-115 ℃;1H NMR(600 MHz, CDCl3):δ8.33(d,J= 13.7 Hz, 1H), 7.58(d,J= 13.7 Hz, 1H), 7.53(t,J= 5.5 Hz, 2H), 7.33(dd,J= 8.4, 1.8 Hz, 1H).
(E)-1-bromo-2-(2-nitrovinyl)benzene(2h)[20]: yield 85%; mp 86-89 ℃;1H NMR(600 MHz, CDCl3):δ8.39(d,J= 13.6 Hz, 1H), 7.69(d,J= 1.0 Hz, 1H), 7.68(d,J= 1.1 Hz, 1H), 7.58(d,J=1.6 Hz, 1H), 7.57(d,J= 1.6 Hz, 1H), 7.54(s, 1H), 7.52(s, 1H), 7.38(td,J= 7.6, 0.8 Hz, 1H), 7.34(td,J= 7.7, 1.7 Hz, 1H).
(E)-1-bromo-3-(2-nitrovinyl)benzene(2i)[20]: yield 83%; mp 58-60℃;1H NMR(600 MHz, CDCl3):δ7.92(d,J= 13.7 Hz, 1H), 7.69(t,J= 1.7 Hz, 1H), 7.62(ddd,J= 8.0, 1.8, 0.9 Hz, 1H), 7.56(d,J= 13.7 Hz, 1H), 7.48(d,J= 7.8 Hz, 1H), 7.34(t,J= 7.9 Hz, 1H).
(E)-1-bromo-4-(2-nitrovinyl)benzene(2j)[20]:yield 93%; mp 150-153 ℃;1H NMR(600 MHz, CDCl3):δ7.94(d,J= 13.7 Hz, 1H), 7.58(dd,J= 10.8, 7.7 Hz, 3H), 7.42(d,J= 8.3 Hz, 2H).
1.4 General procedure for the recovery of 2-hydroxyethyl ammonium acetate
After addition of water to quench the reaction and filtration of the precipitate, the insoluble solid was removed, and 2-hydroxyethyl ammonium acetate could be recovered by removing water from the filtrate and reused directly without further purification.
2 Results and discussion
At the initial step of the development of environmentally benign Henry condensation, a model reaction of benzaldehyde (1a) and nitromethane catalyzed by 2-hydroxyethyl ammonium acetate was used for optimization. The reaction was firstly carried out in water and ethanol as “green” solvents at 80 ℃ resulting 72% and 76% yields, correspondingly(Tab.1, entries 1 and 2). However, it was accidently found that higher yields were obtained by evaporation of the solvent. This observation leads to the conclusion that the reaction might proceed upon solvent removal.
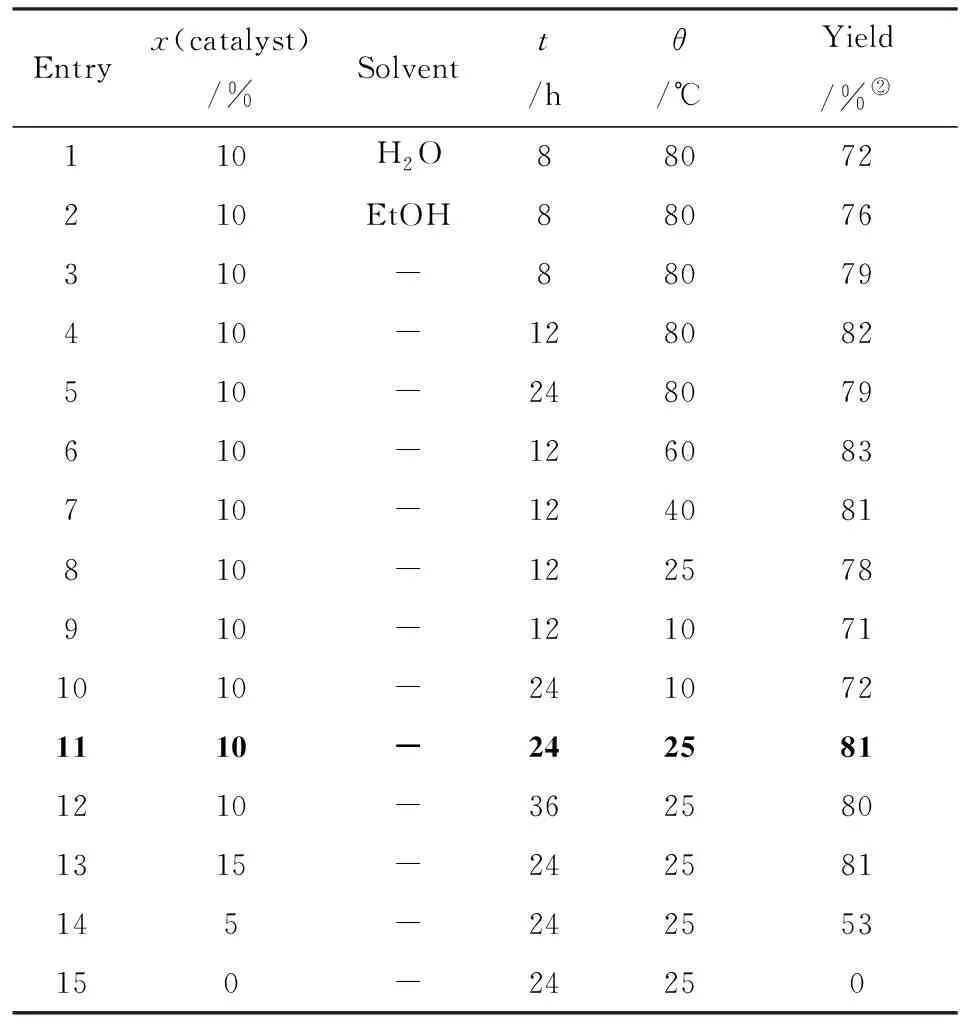
Tab.1 Optimization of the reaction conditions(1) Reaction conditions: benzaldehyde(5 mmol), nitromethane(6 mmol); 表1 反應(yīng)條件的優(yōu)化
To test this hypothesis, a new protocol was developed by direct mixing all the reagents:(1a), nitromethane, and 2-hydroxyethyl ammonium acetate under solvent-free conditions. Then the reaction conditions, such as temperature, time, and catalyst loading were be also optimized. At first, the temperature was varied between 80 ℃ and 10 ℃(entries 3-7) and it was found that higher temperatures increased the condensation rate of (1a) and nitromethane, however, long reaction time decreased the yield, which may be related with side reactions taking place at high temperature(entries 4-8). At the temperatures below 25 ℃, a low conversion resulted in (2a) in only 71% yield even upon extended reaction time(entries 9, 10).
Considering the fact that the reaction proceeds smoothly at lower temperatures, the reaction at 25 ℃ by increasing the reaction time to ensure the full conversion was further examined. It was found that this reaction could be completed after 24 hours with 81% isolated yield(entry 11), and extended reaction time could not improve the yield(entry 12). Additionally, the loading of 2-hydroxyethyl ammonium acetate was also investigated(entries 13-15). It was found that this reaction could not proceed without 2-hydroxyethyl ammonium acetate(entry 15), while 10% mmol loading of 2-hydroxyethyl ammonium acetate is optimal for this reaction, as further increasing the catalyst amount does not improve the yield(entry 13). Hence, this type of condensation reaction can be effectively performed in the environmentally benign conditions(solvent-free and room temperature) in term of hazardousness and energy saving. With the optimized conditions in hand, the reactions of aromatic aldehydes and nitromethane were carried out in presence of 2-hydroxyethyl ammonium acetate under solvent-free conditions, and the results were listed in Tab.2. From Tab.2, it seemed that under the optimized conditions, all the reactions provide corresponding products in good yields regardless of the substrates with electron-donating or electron-withdrawing group.
To investigate the scope of the solvent-free Henry condensation, various aromatic aldehydes were screened by using the optimized reaction conditions found for1a, with the results listed in Tab.2. The overall high conversion of the aromatic aldehydes was achieved at room temperature after 24 h and there was no obvious difference between the electron-withdrawing and electron-donating groups at the ortho-, meta- or para-position of the benzaldehyde. These results indicated that the basicity of 2-hydroxyethyl ammonium acetate is strong enough to efficiently promote the condensation process. All the reactions gave the corresponding products in good to excellent yields(up to 93%) regardless of the substrate structures.
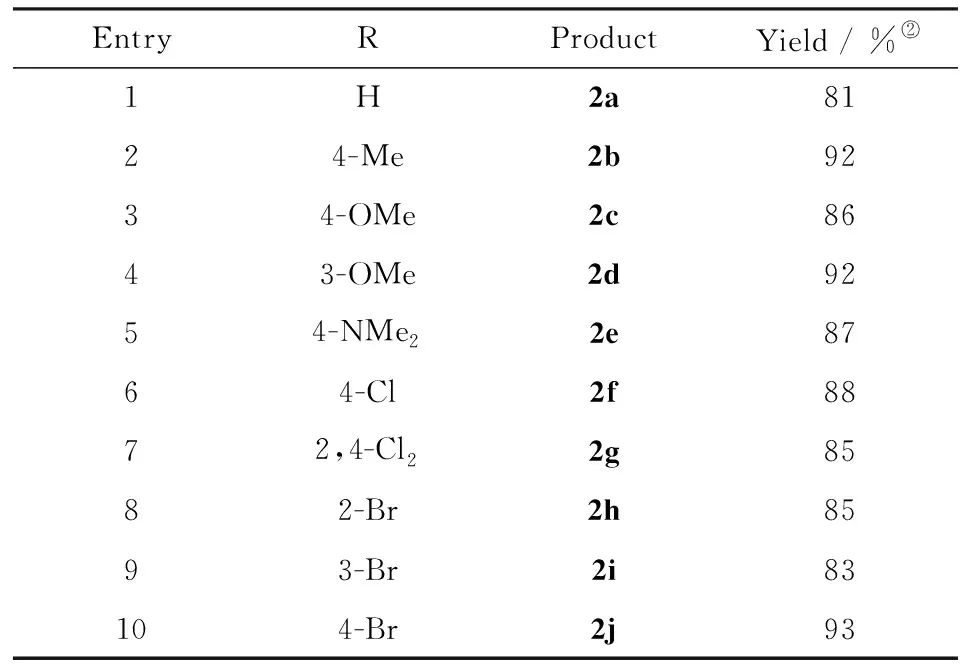
Tab.2 Henry condensation of aromatic aldehydes with nitromethane catalyzed by 2-hydroxyethylammonium acetate under solvent-free conditions(2) Reaction conditions: aldehydes(5 mmol), nitromethane(6 mmol), 2-hydroxyethyl ammonium acetate(0.5 mmol), 25 ℃, 24 h; 表2 無(wú)溶劑條件下乙醇胺乙酸鹽催化芳香醛和硝基甲烷的Henry縮合反應(yīng)
It is well-known that one of the most important principles of “green” processes is a recyclability of the catalyst[10]. Remarkably, it was found that 2-hydroxyethyl ammonium acetate could be quantitatively recovered after a simple filtration and water removal, and it was reused to catalyze the reaction without significant loss of the catalytic activity for three recycles(Tab.3).
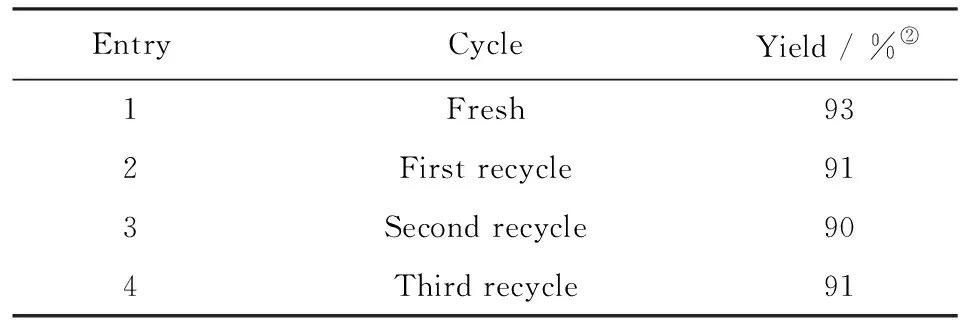
Tab.3 Recyclability of 2-hydroxyethyl ammonium acetate upon reaction of p-bromobenzaldehyde and nitromethane(3)The reaction was carried out at the optimized conditions: 25 ℃, 24 h, 10% catalyst; 表3 乙醇胺乙酸鹽循環(huán)催化對(duì)溴苯甲醛和硝基甲烷的縮合反應(yīng)
3 Conclusion
In summary, a “green” version of the Henry condensation by using functionalized ionic liquid as a catalyst under solvent-free conditions was successfully developed. The use of hazardous acids or bases as catalysts and organic solvents was prevented. Additionally, the functionalized ionic liquid could be readily recovered and reused by a simple treatment without significant loss of the catalytic activity up to three recycles. Furthermore, this reaction proceeded readily at room temperature to ensure good to excellent yields of the correspondingβ-nitrostyrene derivatives in an efficient and environmentally benign procedure.
 中南民族大學(xué)學(xué)報(bào)(自然科學(xué)版)2021年6期
中南民族大學(xué)學(xué)報(bào)(自然科學(xué)版)2021年6期
- 中南民族大學(xué)學(xué)報(bào)(自然科學(xué)版)的其它文章
- 基于Tobit模型的大學(xué)生信用消費(fèi)分析研究
- 對(duì)口幫扶政策下連南瑤族自治縣經(jīng)濟(jì)內(nèi)生發(fā)展動(dòng)力實(shí)證分析
- 首烏藤治療阿爾茨海默病的網(wǎng)絡(luò)藥理學(xué)研究
- 配合物Fe(L)2(PF6)2的合成及其對(duì)部分過(guò)渡金屬離子熒光選擇
- 一類非線性的帶有變時(shí)滯的隨機(jī)微分方程的數(shù)值分析
- 給定懸掛點(diǎn)數(shù)和關(guān)聯(lián)Q-譜半徑最大的k一致超圖的結(jié)構(gòu)
