Dissolution Properties of DINA in DMSO
XING Xiao-ling,ZHAO Feng-qi,JI Yue-ping,XIAO Li-bai,XU Si-yu,HU Rong-zu
(National Key Lab of Science and Technology on Combustion and Explosion,Xi′an Modern Chemistry Research Institute,Xi′an 710065,China)
Introduction
The main component NC(Nitrocellulose)in double-base or composite modified double-base propellant can not possess thermoplastic sufficiently only by the temperature rises,and certain amount of NG(nitroglycerine)must be added as plasticizer to improve the properties of the propellant.DINA is an assistance plasticizer added to solid propellant w ith NG as a classical plasticizer.The low melting point (49-51 ℃)of DINA provides a characteristic for the application of it in propellant area.The group of -NO2connecting w ith -N has higher energy than that connecting w ith -O.
DINA can easily dissolve in anhydride,acetate,benzene,ether and DM SO(dimethyl sulfoxide).In these solvents,DM SO is the very suitable one for the application[1].The solubility of DINA in many solvents has been investigated,but the dissolution process in DMSO has rarely reported until now .In this work,the enthalpies of dissolution of DINA in DMSO are measured by means of a calvet microcalorimeter,the enthalpies[2-3]of DINA in DMSO are studied intensively,and it is expected that this will be useful for its industrial applications.
1 Experimental
1.1 Materials
DINA used in the experiment w as prepared by Xi′an M odern Chemistry Research Institute,its purity w as more than 99.5%.DMSO(m .p.18-20;=1.098-1.102)used as solvent w as of analytical purity.
1.2 Equipment and conditions
All measurements w ere made using a RD496-2000 Calvet microcalorimeter.T he enthalpy of dissolution of KCl(spectrum purity)in distilled w ater measured by RD496 -2000 Calvet microcalorimeter at 298.15 K was 17.234 kJ/mol,and the relative error w as less than 0.04%compared w ith the literature value[4]17.241 kJ/mol.This show ed that the device for measuring the enthalpy used in this w ork w as reliable.
The enthalpies of dissolution were measured at 302.15,306.15,310.15 and 313.15K,respectively.
2 Results and discussions
2.1 The relationships of the concentration and enthalpies of dissolution in DMSO for DINA at different temperatures
Each of the dissolving process under four different temperatures for DINA in DM SO is endothermic,and the experimental and calculated values[5]of enthalpies of dissolution in DM SO for DINA are given in the Table 1 .In Table 1 ,b represents the concentration of the solution after the dissolution,ΔdissH is the enthalpies of dissolution in DMSO for DINA.
The relationships of ΔdissH vs.b1/2at different temperatures are show n in Fig.1 .From Fig.1 ,one can see that the Δdiss H changes regularly w ith the square values of the concentration of DINA after the dissolution process.With the temperature rising,the absolute values of enthalpy of dissolution increase when the concentrations of DINA in DMSO are the same.
By substituting the values of b in Table 1 into Eq.(1),the empirical formulae of enthalpy of dicsolution ΔdissH at different temperatures are obtained and listed as Table 2.
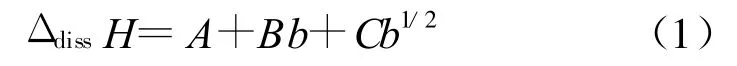
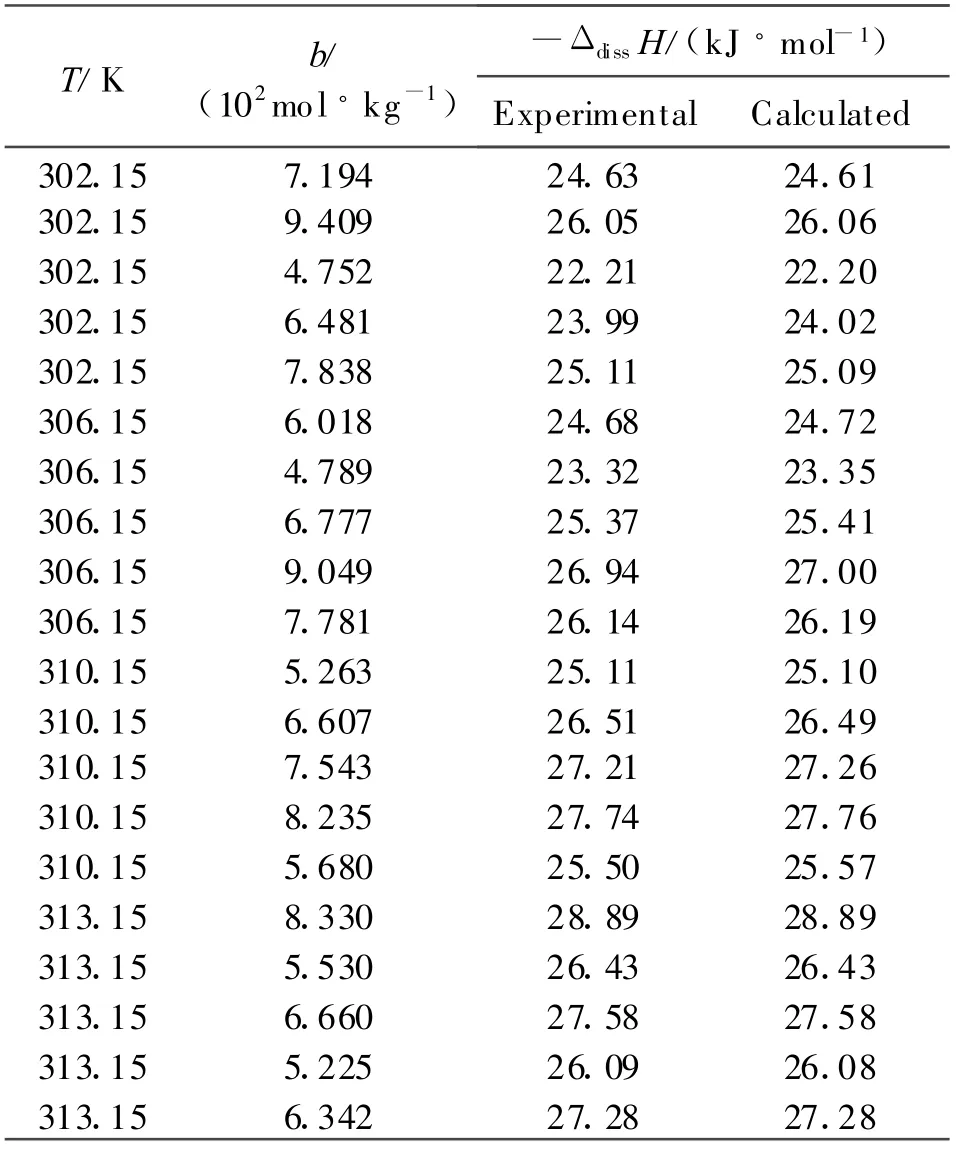
Table 1 The enthalpies of dissolution of DINA in DMSO at different temperatures
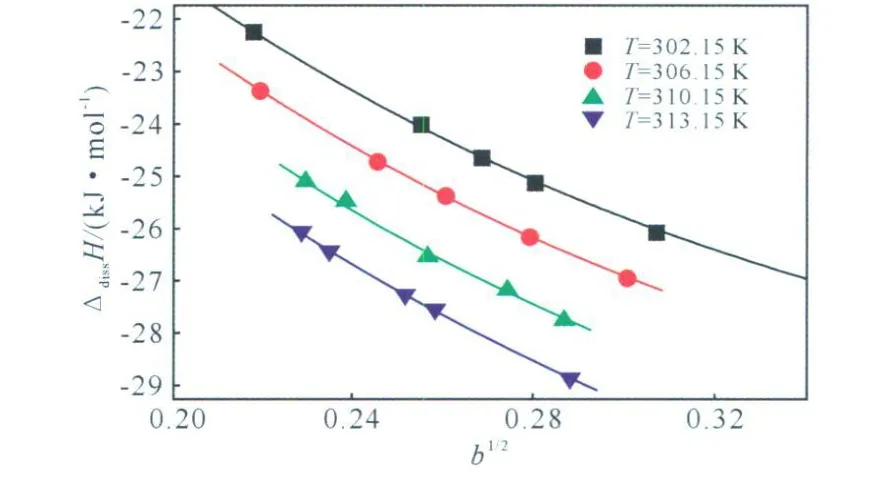
Fig.1 The relationships of ΔdissH vs.b1/2at different temperatures
Where A,B and C are coefficients.
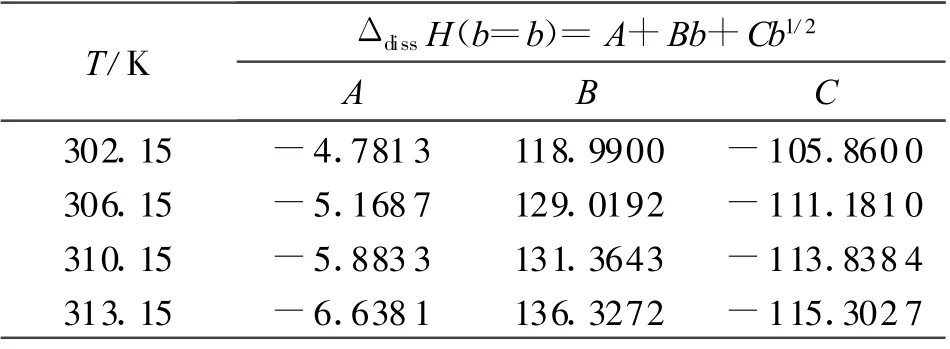
Table 2 The empirical formulae of enthalpy ΔdissH
2.2 The method estimating the enthalpies of dissolution in DMSO for DINA at random temperatures
The values of A,B and C change w ith the variety of the temperatures as show n in Fig .2,the polynomial formulae (2)-(4)can describe the regularly change at different temperatures,respectively .r is the correlatilon coefficient.
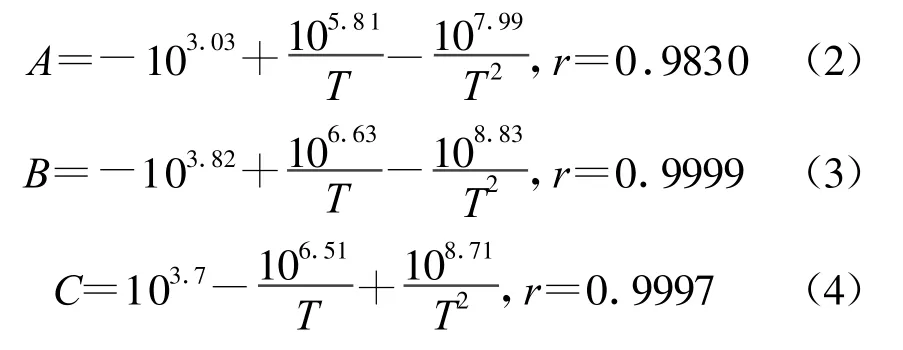
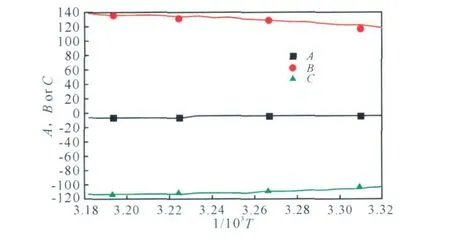
Fig.2 The values of A,B and C vs.1/T
Based on Eq.(2)-(4),one can obtain the corresponding values of A,B and C at each temperatures,then the enthalpies[6]of dissolution in DMSO for DINA (ΔdissH)can be acquired.If the effect of the concentration (b)of the solution can be neglected,the relationship of ΔdissHmeanvs.T is show n as Fig.3 .ΔdissHmeanis the average value of the ΔdissH at the same temperature.
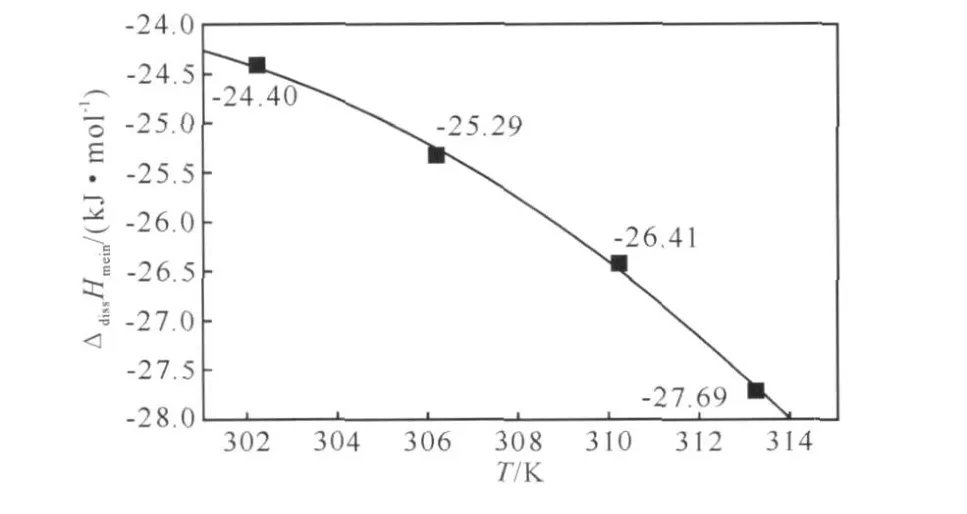
Fig.3 The relationship of ΔdissHmeanvs.T
Then we get Eq.(5)describing the variety of ΔdissHmeanw ith the temperatures.According to the Eq(5),the average values of enthalpies of dissolution in DMSO for DINA (ΔdissHmean)at different temperatures(the temperatures must not be faraw ay from our experiment temperature range)can be obtained easily.This is very meaningful for the application of DINA in industrial techniques (almost in our experiment temperature range).
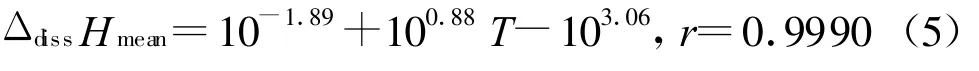
3 Conclusions
(1)The enthalpies of dissolution of DINA in DM SO measured by means of a calvet microcalorimeter at 302.15,306.15,310.15 and 313.15K,correspondingly,empirical formulae for the calculation of the enthalpies of dissolution at four different temperatures were ΔdissH =-4.781 3+118.99b-105.86b1/2,ΔdissH =-6.168 7 +129.0192b -111.181 3b1/2,ΔdissH =-5.883 3 +131.364 3b -113.838 4b1/2and ΔdissH=-6.638 1+136.327 2b-115.302 7b1/2,respectively.
(2)All of the coefficients of A,B and C at different temperatures vary according to polynomial equationsspectively .This is very helpful for the acquisition of the enthalpies of dissolution in DMSO for DINA at other unexperimental temperatures.
(3)The average values of enthalpies of dissolution in DMSO for DIN A vs.temperatures can be described as ΔdissHmean=-10-1.89T2+100.88T -103.06,then one can obtain the enthalpies of dissolution in DMSO for DINA at any temperature.
[1]XING Xiao-ling,XUE Liang,ZH AO Feng-qi,et al.Thermochemical properties of 1,1-diamino-2,2-dinitroethylene(FOX-7)in dimethyl sulfoxide(DMSO)[J].Thermochimica Acta,2009,35:491-495.
[2]GAO H ong-xu,ZHAO Feng-qi,H U Rong-zu.Differential and integral isoconversional non-linear methods and their application to energetic materials[J].Chin J Chem,2008,26:1973-1978.
[3]H U Rong-zu,SH I Qi-zhen.Thermal analysis of kinetics[M].Beijing:Science Press,2001.
[4]M anthada V K.The enthalpy of solution of S RM 165(kd)in H2O[J].Jounnal of Research of the National Bureau stan dands,1980,85(15):467-480.
[5]XING Xiao-ling,XUE Liang,ZHAO Feng-qi,et al.Theamochemical pnopenties of 1,1-diamino-2,2-dinitothylene(FOX-7)in dimehtyl sulfoxide(DMSO)[J].Thenmochimica Acta,2009,491:35-38.
[6]XING Xiao-ling,XUE Liang,ZH AO Feng-qi,et al.Dissolution properties of the CL-20 in ethyl acetate and acetone[J].J Therm Anal Calorim,2010,99:703-707.

