Safety and immunogenicity of COVID-19 vaccination among liver transplant recipients in China
Qiu-Ju Tin , b,# , Mn Xi ,# , Ji-To Wn ,# , Yi Wn , Bi Zhn , Jin-Zhn Ci , b ,Xio-Lon Qi , Wi Ro , b, ?
a Division of Hepatology, Liver Disease Center, The Affiliated Hospital of Qingdao University, Qingdao 2660 0 0, China
b Department of Organ Transplantation, The Affiliated Hospital of Qingdao University, Qingdao 2660 0 0, China
c Department of Gastroenterology, The Affiliated Hospital of Qingdao University, Qingdao 2660 0 0, China
d CHESS-COVID-19 Group, Xingtai People’s Hospital, Xingtai 0540 0 0, China
e Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao 2660 0 0, China
f Department of Immunology, Qingdao University Medical College, Qingdao 2660 0 0, China
g CHESS Center, Institute of Portal Hypertension, The First Hospital of Lanzhou University, Lanzhou 730 0 0 0, China
To the Editor:
During the ongoing coronavirus disease 2019 (COVID-19) pandemic globally, patients with chronic liver diseases (CLD), particularly cirrhosis, hepatobiliary malignancies, candidates for liver transplantation (LT), and immunosuppressed LT recipients appear to be at increased risk of infections, which leads to an increase in mortality [1–6] . Apart from physical distancing, quarantine and isolation, vaccination is crucial for the restraining of the epidemic and the protection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and aggravation of COVID-19 [ 7, 8].A recent prospective, multicenter, open-label study in China has demonstrated the safety of inactivated whole-virion SARS-CoV-2 vaccines in patients with CLD; however, these patients had decreased response (76.7%-78.9%) to SARS-CoV-2 vaccines compared with healthy subjects (90.3%), which might be associated with the impairment in immunity in the population with CLD [9] . This raises the concern in the safety and immunogenicity of COVID-19 vaccination among LT recipients in China since this population may have more suppressed immunity [ 8 , 10 , 11 ]. Herein, we preliminary investigated the influence of COVID-19 vaccination on general safety, graft function, host immunogenicity of vaccination among LT recipients in a large transplantation center in China.
In this single-center cohort study, adult LT recipients regularly followed up with normal graft function at the Affiliated Hospital of Qingdao University from December 2020 to July 2021 were investigated, and those completing the full COVID-19 vaccination schedule (two doses from Beijing Institute or three doses from Anhui Institute) were included. Data were expressed as median (interquartile range) or number (percentage).
In total, 62 LT recipients were included. The median age of these participants was 55 years, and the majority were males(n= 53, 85.5%) and Chinese Han population (n= 60, 96.8%). The median time from LT to the first vaccination was 49.8 (28.3-81.6)months. The most common indications of LT were hepatocellular carcinoma (n= 28, 45.2%). Chronic hepatitis B infection was the most common etiology of liver disease (n= 45, 72.6%).
Fifty-seven (91.9%) participants received the inactivated vaccine(two doses from Beijing Institute) and 5 (8.1%) cases of recombined vaccine (three doses from Anhui Institute). Local site reactions included pain at the injection site (13/62 after the first dose),swelling (2/62 after the first dose) and pruritus (1/62 after the second dose). Systemic reactions such as fever (4/62) were uncommon, although more-than-baseline fatigue was reported by 3/62 and 4/62 cases after the first and second dose, respectively. During the follow-up period, LT recipients experienced no events of suspected or confirmed graft rejection, compromised graft function,neurological events, nor severe allergic reaction.
The liver and kidney function tests prior to and after the full doses of SARS-CoV-2 vaccination were assessed ( Table 1 ). The overall serum levels of liver and kidney function indicators were all comparable and within the normal range.
Tacrolimus was used as the backbone of the immunosuppressive regimen in 51 (82.3%) cases. Sirolimus was used in 11 cases (17.7%), mostly in combination with antimetabolic drugs or tacrolimus (5 cases and 4 cases, respectively). For the 51 cases taking tacrolimus, the blood levels of drug concentration were comparable before [4.30 (3.10-6.45) ng/mL] and after [3.75 (3.05-5.96) ng/mL] the vaccination (P= 0.632). For the 11 cases taking sirolimus, they also had comparable blood levels of sirolimus concentration before and after vaccination [3.92 (3.57-4.31) vs. 3.63(3.03-6.80) ng/mL,P= 0.668].
To evaluate the influence of COVID-19 vaccination on the immune state in LT recipients, the changes of the immune cells including myeloid cell lineage and lymphocyte lineage were examined ( Table 1 ). For the myeloid cell lineage, eosinophil count wasdecreased significantly (from 0.1 × 109to 0.07 × 109); however,the overall indexes were within the normal range. For the lymphocyte lineage, CD3 + HLADR + activated T cell (3.60% vs. 2.50%),and CD3-HLADR + activated B/NK cells (11.50% vs. 8.80%) were decreased significantly while the CD3 + HLADR- inactivated T cells(64.60% vs. 67.35%) were increased significantly. Similar with the myeloid cell lineage, they were all within normal range despite of the changes.
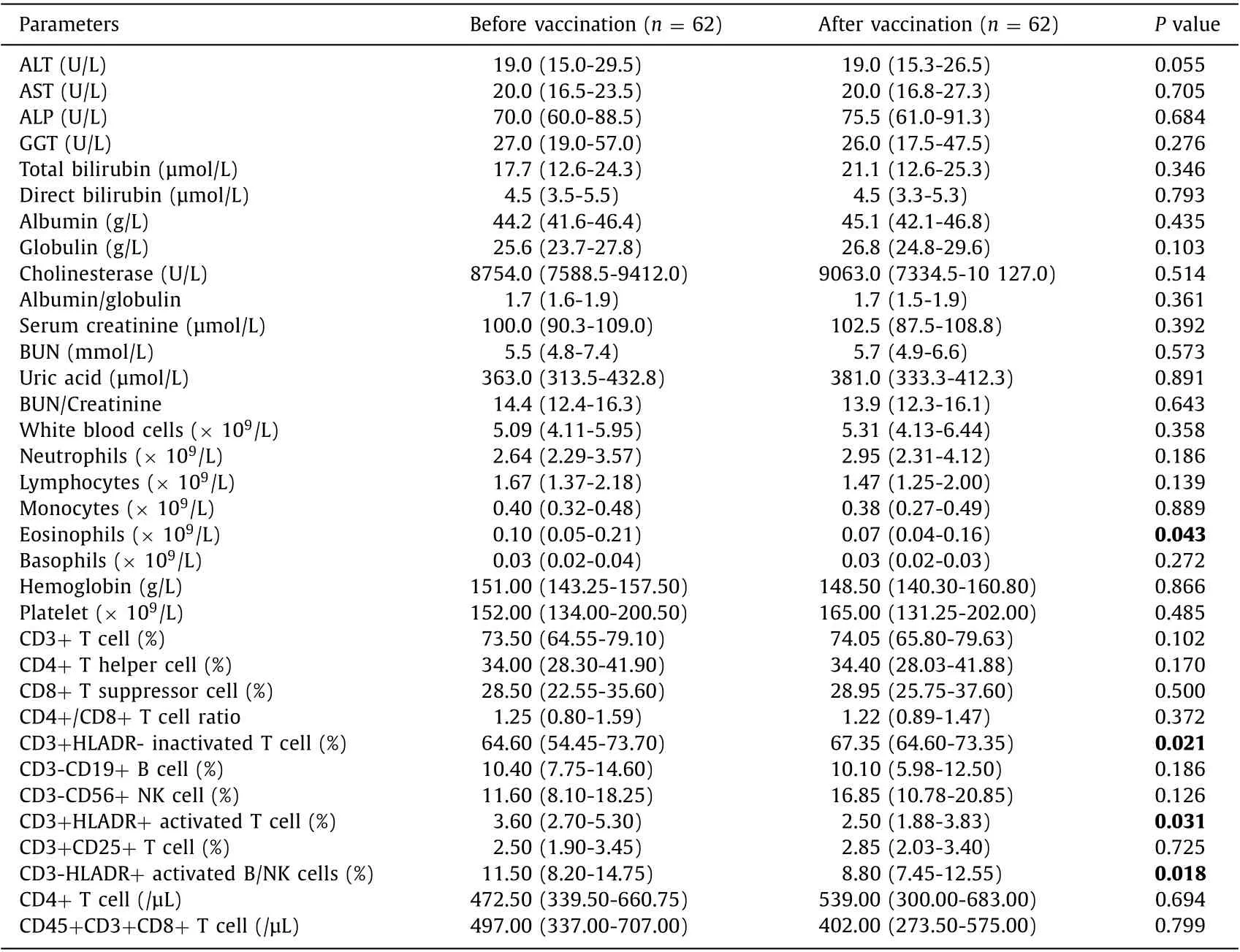
Table 1 Liver, kidney function test and immune status assessment prior to and after the full doses of COVID-19 vaccination in liver transplantation recipients.
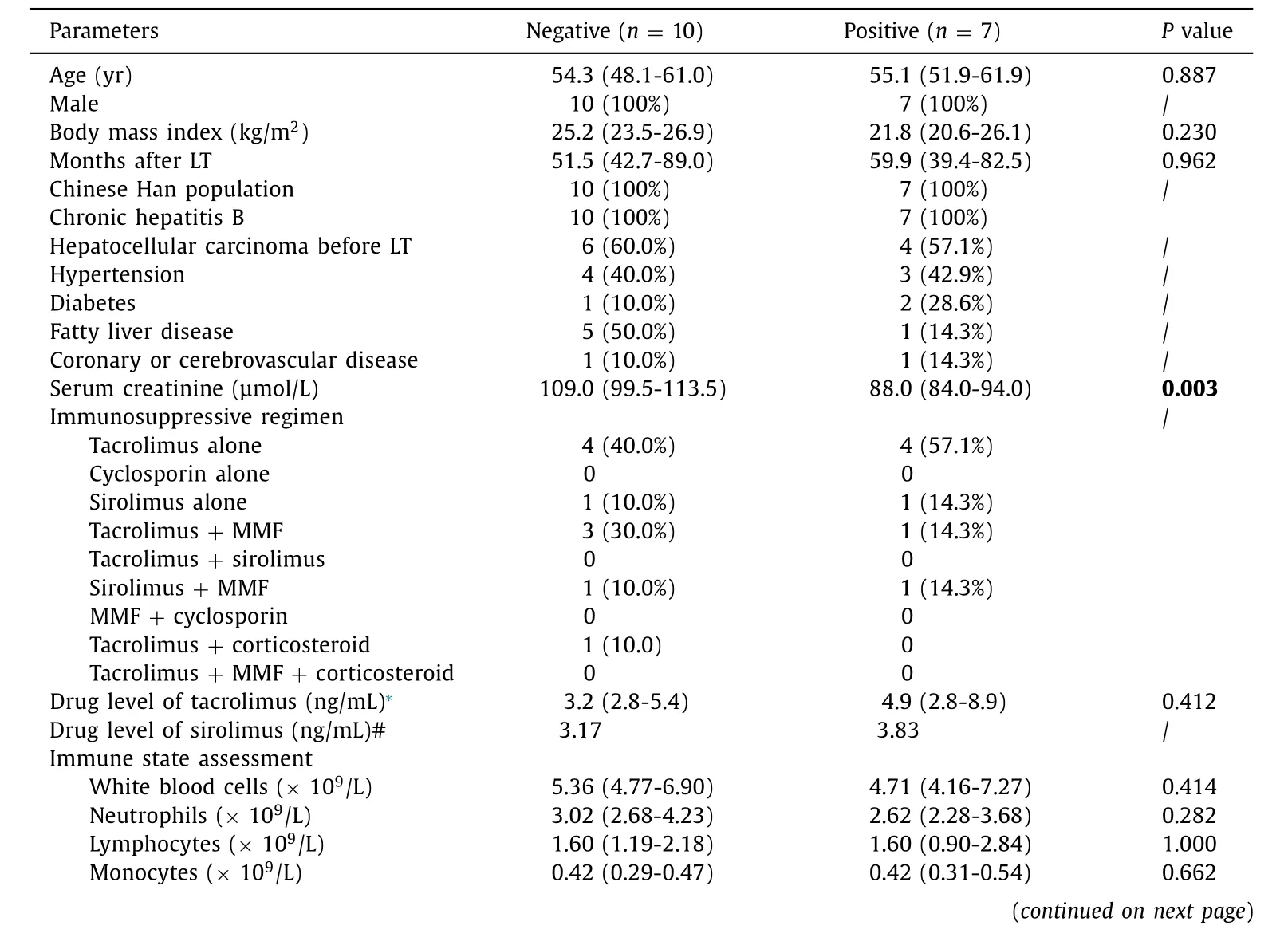
Table 2 Comparison of the clinical and laboratory data for the LT recipients with positive and negative serological response.
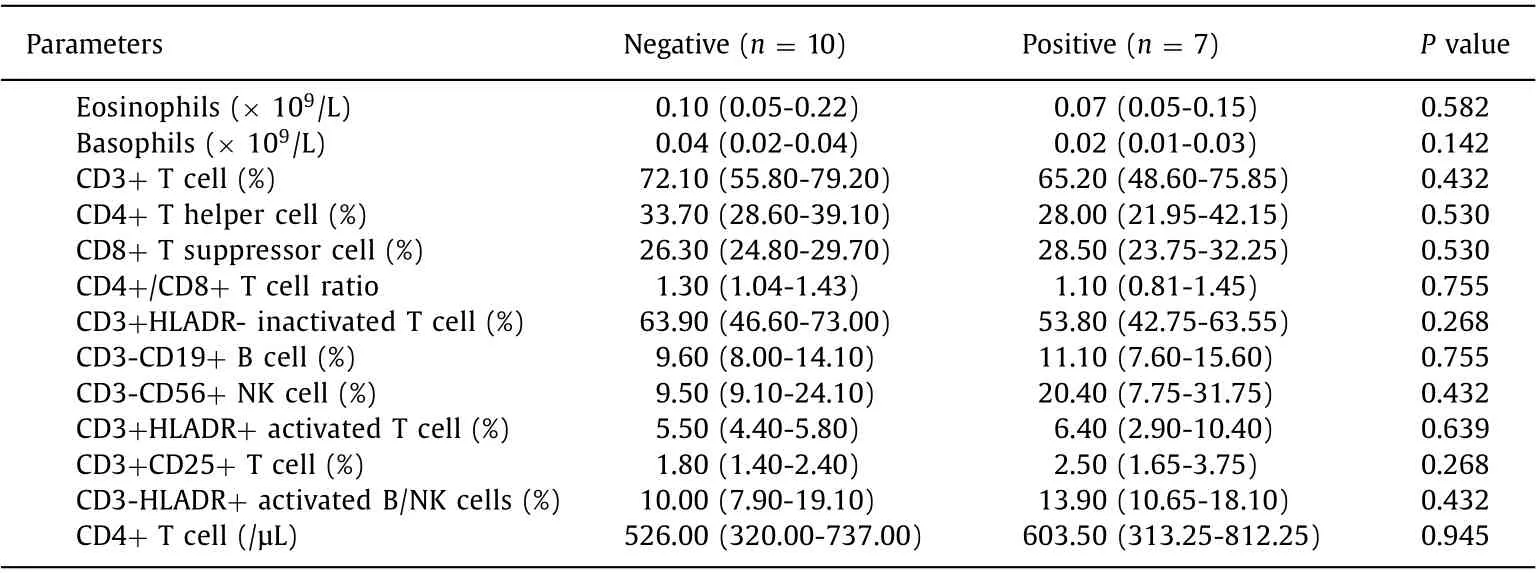
Table 2 ( continued )
Of the 62 LT recipients, 17 had blood samples taken 10-20 days after the full vaccination. Seven (41.2%) cases had positive serology of neutralizing total antibody against the COVID-19(nCoVNTAb) [9] , with a median level of 13.7 (12.8-29.2 AU/mL)AU/mL. The response rate was similar to the previous study of mRNA SARS-CoV-2 vaccine among LT recipients [11] , yet lower than that reported in patients with CLD in China [ 7 , 9 ].
The comparison of the clinical and laboratory data of LT recipients with positive and negative nCoVNTAb is presented in Table 2 .Although within the normal range, participants with negative serology had significantly higher serum creatinine: 109.0 (99.5-113.5) μmol/L in the negative serology group vs. 88.0 (84.0-94.0)μmol/L in the positive serology group (P= 0.003). This is in line with the recent reports that impaired renal function was associated with a negative response to SARS-CoV-2 vaccine among solid organ transplantation recipients [ 11 , 12 ]. Comparisons of tacrolimus or sirolimus level, time from transplant, sex, body mass index, etiology of liver disease or comorbidities did not yield statistically significant differences in immunogenicity. No significant change of immune state was found before and after the vaccination. By multivariate analysis, no factors were identified as significantly associated with negative serological response, which might largely be attributed to the small sample size taking the COVID-19 antibody test.
In conclusion, the COVID-19 vaccination in LT recipients is safe.We would like to reiterate our strong recommendation to vaccinate in this population even if immunocompromised patients may yield a decreased response to vaccination. Future studies should address interventions to improve vaccine responses in this population, including additional booster doses or immunosuppression modulation.
Acknowledgments
None.
CRediT authorship contribution statement
Qiu-Ju Tian: Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft. Man Xie: Data curation, Formal analysis, Methodology, Software, Writing – original draft. Ji-Tao Wang: Data curation, Formal analysis, Methodology.Yi Wang: Data curation, Investigation, Project administration, Validation. Bei Zhang: Formal analysis, Investigation, Writing – review & editing. Jin-Zhen Cai: Conceptualization, Methodology, Resources, Writing – review & editing. Xiao-Long Qi: Conceptualization, Methodology, Writing – review & editing. Wei Rao: Conceptualization, Funding acquisition, Resources, Supervision, Writing –review & editing.
Funding
This study was supported by grants from the "Clinic + X" of the Affiliated Hospital of Qingdao University (No. 3754) and Social Science Popularization and Application Research Project of Shandong Province (No. 2021-SKZC-18).
Ethical approval
This study was approved by the Institutional Ethical Review Board of the Affiliated Hospital of Qingdao University (QYFYKYLL 994311920). Written informed consents were obtained from all participants.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2022年6期
Hepatobiliary & Pancreatic Diseases International2022年6期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- YAP activates pancreatic stellate cells and enhances pancreatic fibrosi s
- Combined analysis of imaging tumor capsule with imaging tumor size guides the width of resection margin for solitary hepatocellular carcinoma
- Prediction of early recurrence of hepatocellular carcinoma after liver transplantation based on computed tomography radiomics nomogram
- Complications of modern pancreaticoduodenectomy: A systematic review and meta-analysis
- Technical aspects in pancreaticoduodenectomy and therapeutic strategies for pancreatic cancer: History, current status, and future perspectives
- Contrast-enhanced ultrasound predicts microvascular invasion in patients with hepatocellular carcinoma
