Excited-State Dynamics of Large-Sized Thiolate-Protected Au n(n>100)Nanoclusters
ZHOU Meng
University of Science and Technology of China,Hefei,230026,China
Abstract:Gold nanoclusters(size<2.2 nm)bridge metallic gold nanoparticles and single gold atoms,playing a crucial role in revealing the origin of surface plasmon resonance and metallic bonding.Ligand-protected gold nanoclusters provide a suitable platform to understand metal to non-metal transitions while the excited-state dynamics of gold nanoclusters in the transition regime remain elusive. In this minireview,I summarize the excite-state dynamics of large-sized Au n(n>100)nanoclusters and compare them with those of plasmonic gold nanoparticles.By looking into the electron and vibration dynamics of a series of thiolate-protected gold nanoclusters in the transition regime,the electronic structures of these nanoclusters are further discussed.A deeper understanding of the mechanism of electron relaxation and energy dissipation of large-sized gold nanoclusters will be of great help to connect the optical properties of metal clusters and nanoparticles,which will further promote the design of those functional nanomaterials.
K ey wor d s:gold nanoclusters;excited-state dynamics;electronic structure;surface plasmon resonance
CONTENTS
I.Introduction 17
II.Electronic structure 18
III.Photophysics 19
IV.Excited-state dynamics of large-sized gold(n>100)NCs 20
V.Conclusion and outlook 23
Acknowledgments 23
References 23
I.INTRODUCTION
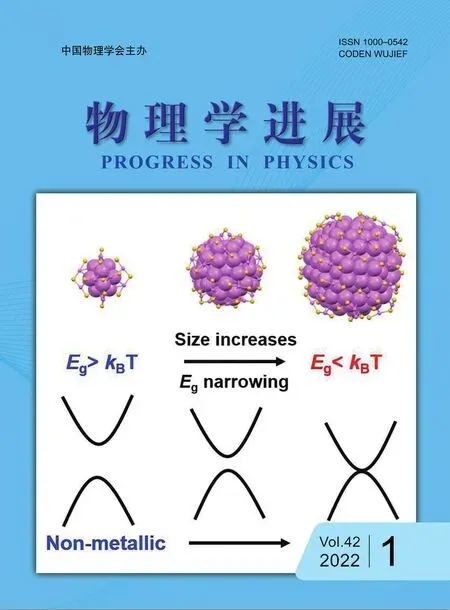
The evolution from metallic to non-metallic behaviors in gold nanoparticles is a fundamental question,which calls for both experimental and theoretical efforts[1-5].Ligand-protected gold nanoclusters(NCs)consist of tens to hundreds of gold atoms,which have attracted considerable research interests in both fundamental science and applications[6-24]. In contrast with large plasmonic gold nanoparticles(NPs),ultrasmall(diameter smaller than 2.2 nm)gold NCs exhibit molecular-like properties including discrete energy levels and multiple absorption bands in their UV-vis absorption spectra[25,26].When thesizeof AunNCsincreases(typicallyn>100),theenergy levelswill gradually become crowded and f inally turn quasi-continuous,which marks the transition from molecular state to metallic state(Figure 1)[2].Therefore,understanding the photo-physics of these large sized Aun(n>100)NCs is important for revealing the evolution of metallic state.

FIG.1.Energy gap versus size from non-metallic to metallic gold nanoclusters.
Based on a previous study,small and mediumsized thiolate-protected Aun(n<50)NCs exhibit non-scalable optical properties[27]. Their UVvis absorptions and excited-state dynamics are highly dependent on their structure and compositions,showing little size dependence[28].Large sized Aun(n>100)NCs,on theother hand,exhibit strong sizedependence in their HOMO-LUMO gap(Eg),excited-state lifetimes and other optical properties[29-33].By comparing the relaxation dynamics of large sized Au NCs and metallic Au NPs,it helps to deepen the understanding of metal to nonmetal transition.Sincemost of thoselargesized Au NCsarenon-luminescent,transient absorption is more suitable than transient f luorescence for probing their excited-state dynamics.The steadystate UV-vis absorptions of large sized Au NCs are broadened and featureless,while the transient absorptions measured by the pump-probe experiment show multiple ground state bleaching peaks.Therefore,it is more convenient to track the evolution of optical properties by looking into their excited-state properties[34].
In this minireview,I focuse on the excite-state relaxation dynamics of several large sized Au NCs.I will f irst introduce the different electronic structures and optical properties of metallic Au NPs and nonmetallic Au NCs.In the second section,I will discuss the photo-physics of both Au NCs and NPs by comparing their optical absorptions,excited-state behaviors and relaxation pathways.In the third part,Iwill introduce the excited-state dynamics of several molecularlike Aun(n>100)NCs and summarize a simple rule to understand their behaviors.Finally,I will conclude and provide our outlook for the future directions on this topic.
II.ELECTRONIC STRUCTURE
In bulk gold,a very high electron density givesrise to densely spaced electronic state and quasi-continuous bands[1].The free-electron or Drude theory can well describe the 6selectrons in gold[1].Gold NPs in the size range of 3 and 100 nm exhibit surface plasmon resonance(SPR)peak in their UV-vis absorption spectra,which stimulate considerable research interests in fundamental science and various applications[35,36].The SPR peak originates from the collective excitation of conduction band electronsand the spectral features are highly dependent on their size,shapeand thesurrounding environment[37,38].The spectra of metal NPs can be precisely calculated by Mie and Gan’s theory[39,40].When the size of gold NPs increases from 2.5 to 60 nm,the SPR peak becomes stronger and experience redshift and sharpening(Figure 2a)[1].When the size further increases from 60 to 120 nm,the SPR peak becomes boadened again.The linewidth broadening of the SPR peak is due to the radiation damping at larger sizes and electron surface scattering at small sizes[1].As the size of Au NPs decreases,the number of gold atoms in the nanoparticle will become f inite,and the spacing(δ)of the electronic energy level will increase accordingly.At some size,when the energy spacing of Au cluster is close to the thermal energy at room temperature(kBT=0.02 eV),the energy quantization will become important and the particle can no longer support the SPR,which marks the emergence of a nonmetallic state[4].

FIG.2. (a)Extinction spectra of different sized Au NPs in water.Reproduced with permission from ref.[1].Copyright 2011 American Chemical Society.(b)UV-vis absorption spectrum of[Au25(SR)18]-NCs dissolved in toluene.Reproduced with permission from ref.[26].Copyright 2008 American Chemical Society.
In molecular-like Au NCs,the number of gold atoms is f inite,which can be determined by solving the crystal structures and measuring their mass spectroscopies[25]. Since these small nanoparticles have discrete energy levels,they possess multiple optical absorption peaks in their UV-vis spectra.Figure 2b shows the UV-vis absorption spectrum of[Au25(SR)18]-NCs and three prominent peaks can be observed between 1.0 and 3.5 eV[26].Unlike the collective excitation in SPR peak in larger Au NPs,the absorption peaks in small Au NCs are singleelectron transitions.Based on theoretical calculations,the molecular orbitals in Au NCs can be simulated,which is similar to those of organic molecules. For example,according to density functional theory(DFT)calculations,the absorption peakain Figure 2b is mainly contributed by the HOMO-LUMO transition of Au25(SR)18,which is almost exclusively from the Au13core[26].By extrapolation of the absorption spectrum to zero,it is possible to estimate the optical HOMOLUMO gap of a nanocluster.The optical absorption of Au NCs is also dependent on their size,shape and compositions[34].More importantly,the crystal structure plays an important role in modifying their optical properties[41],which is comparable to the case in organic molecules.
When the size of Au NCs increases,the absorption peaks will become broadened and the onset of absorption will experience redshift,which marks the narrowing of the HOMO-LUMO gap[27].In those large sized Au NCs(n>100),the UV-vis absorption peak is broadened,and the onset of absorption will fall in the infrared region.When the energy gap is smaller than 0.4 eV,it is difficult to estimate the energy gap from UV-vis absorption[29,42].At some sizes between 2 and 3 nm,all those absorption peaks in the near-infrared region will disappear and SPR peaks will start to dominate the UV-vis absorption[33].
III.PHOTOPHYSICS
Understanding the energy dissipation mechanism in gold nanoparticles and nanoclusters is of great importance for their applications in photo-energy storage and conversion.In the plasmonic gold NPs,photoexcitation will lead to the collective excitation of electrons in the conduction band[1].After the initial ultrafast electron dephasing process(around 10 fs),the excited electron will experience electron-electron scattering(a few 100 fs)to reach an equilibrium.The hot electron will then transfer their energy to the lattice via electron-phonon coupling(e-p coupling)in 1-5 ps[43-46].Finally,the energy is dissipated into the environment via heat exchange with the surrounding medium(Figure 3a).The e-p coupling in Au NPs can be described by the two-temperature model(TTM):[47]

whereTeandTlare the temperature of electron and lattice,respectively.Ce(Te)andClare the heat capacity of electron and lattice,respectively.gis the e-p coupling constant.Since the heat capacity of electronCe(Te)is dependent on initial electron temperature,the energy exchange time between electron and lattice should be dependent on the pump f luence(Figure 3b).According to a previous study,the e-p coupling process is faster for smaller gold and silver NPs[48].When the diameter of Au NPs is smaller than 10 nm,the e-p coupling time will drop signif icantly[48].The f inal heat dissipation process is dependent on particle size,the heat capacity of the surrounding medium as well as the surface protecting ligands[1].In very small Au NPs,the heat dissipation may occur before the e-p coupling process is complete[49].

FIG.3.(A)Transient absorption kinetics probed at different wavelengths of 13 nm Au NPs;(B)Kinetics at different pump f luence of 13 nm Au NPs.Reproduced under a Creative Commons CC-BY license from ref[46].Copyright 2019 MDPI.

FIG.4.(a)Femtosecond and nanosecond transient absorption data map of[Au25(SR)18]-NCs pumped at 400 nm.Reproduced with permission from ref.[51].Copyright 2018 American Chemical Society.(b)Excited-state lifetime versus energy gap of Au NCs.Reproduced with permission from ref.[27].Copyright 2019 American Chemical Society.
Unlike Au NPs,ultra-small Au NCs exhibit a large HOMO-LUMO gap and photoexcitation will induce single-electron excitation,which will pump the electrons from the electronic ground state to the excited-state. In Au25NCs,following photoexcitation,the excited-state will f irst experience ultrafast cooling to the lowest exited state in less than 1 ps(Figure 4a)[34,50,51].With excitation energy near the HOMO-LUMO gap,the ultrafast cooling will disappear and only the long-lived relaxation remains.The ultrafast hot electron cooling is independent on the pump f luence,unlike plasmonic gold NPs.Moreover,the ultrafast cooling disappears when the excitation energy is close to the HOMO-LUMO gap of the nanocluster[51]. Subsequently,the excited-state energy of[Au25(SR)18]-NCs will relax slowly to the ground state via radiative and non-radiative decay(around 100 ns)[51].
The excited-state lifetimes of Au NCs are sensitive to their size,shape,crystal structure and composition[34].In general,when the HOMO-LUMO gap of Au NCs is smaller than 1.0 eV,their excited-state lifetimes will drop signif icantly and follows the energy gap law,that is,a smaller HOMO-LUMO gap will lead to a shorter excited-state lifetime(Figure 4b)[27,52].In rod-shaped Au25NCs,the excited-state lifetime is as long as 2 microseconds[53].20 times longer than that of spherical shaped[Au25(SR)18]-NCs(ca.100 ns).In same-sized Au NCs,a previous study reported that the different atomic packing modescould lead to a variation of three orders of magnitude of carrier lifetimes[23].All these facts indicate that the photo-physics of Au NCs are largely determined by their electronic structures.
IV.EXCITED-STATE DYNAMICSOF LARGE-SIZED GOLD(n>100)NCs
When the size of gold nanocluster is larger than Au100,their optical energy gap will fall below 0.4 eV,and their optical properties will strongly dependent size. Here,I will use Au103S2(SR)41,Au130(SR)50,Au133(SR)52,Au144(SR)60and Au246(SR)80(Au103,Au130,Au133,Au144,Au246for short)as examples to introduce the photo-dynamics of large sized gold nanoclusters.
Au103is the largest nanocluster that the energy gap can be determined from the UV-vis-NIR absorption spectrum and the HOMO-LUMO gap was around 0.42 eV(Figure 5a,b)[29].For those gold NCs larger than Au103,their energy gaps are smaller than 0.4 eV and the lowest energy absorption band is buried in the NIR absorption of surface organic ligands.The excited-state relaxation of Au103can be described by a fast relaxation(2 ps+16.6 ps)from higher excitedstate to lower excited-state followed by a slow relaxation(420 ps)to the ground state(Figure 5c,d)[29].In contrast to those small Au NCs,the excited-state absorption prof iles of higher excited-state are similar to that of lower excited-state,which indicates little spectral evolution.Such an observation should be from the densely spaced higher excited-state,which gives rise to very broad and featureless excited-state absorption overlapped with ground state bleaching.A similar relaxation pathway can be observed in other large sized(n>100)Au NCs such as Au130and Au133[30,31].

FIG.5. (a)UV-vis and(b)NIR absorption spectrum of Au103 dissolved in CCl4;(c)Evolution associated spectra from the global f itting on the transient absorption data of Au103;(d)TA kinetics of Au103 probed at different wavelengths.Reproduced with permission from ref.[29].Copyright 2017 American Chemical Society.
In Au130and Au133,thesteady-state and transient absorption spectra aresimilar to that of Au103whilethe energy gap is too small to be determined from optical measurement(Figure 6a,b)[27].From electrochemical measurements,the energy gaps of Au130and Au133are determined to be around 0.3 and 0.15 eV respectively[30,54].The relaxation dynamics of both Au130and Au133consist of two decay components.The fast decay(around 2 ps for both Au130and Au133)was assigned to the cooling of hot electrons from higher excited-state to lower excited-state[30,31].By changing the excitation energy from 360 nm(3.4 eV)to 1 200 nm(1.03 eV)in Au130,the amplitude of fast relaxation decreased and f inally disappeared,which proved the assignment of the fast relaxation component[30].Theslow decay(130 psfor Au130and 30 psfor Au133)was assigned to the relaxation from the lowest excited-state to the ground state.One can observe that the steady-state and transient absorption spectra are almost identical for Au130and Au133(Figure6a).However,theslow relaxation timeincreasesexponentially as the energy gap decreases from Au130to Au133,which follows the energy gap law of small molecules.Such an observation indicates that despite similar spectral features between Au130and Au133,their photo-physics are largely determined by their energy gap.

FIG.6. (a)UV-vis absorption spectra of Au103,Au130,Au133,Au144,Au246 NCs;(b)TA spectra of f ive NCs probed at 1.0 ps;(c)Comparison of TA kinetics at a selected wavelength of f ive NCs.Reproduced with permission from ref.[27].Copyright 2019 American Chemical Society.
When the size of Au NCs further increases,their energy gaps will be close to the thermal energy(kBT=0.02 eV or 20 meV)at room temperature.For Au144,the energy gap was determined to be 0.17 eV by the electrochemical method[54].In Au246,the gap was so small that even the electrochemical methods cannot resolve it[32].Unlike Au103,Au130and Au133,the excited-state relaxation of Au144and Au246can no longer be described by a fast relaxation followed by a slow relaxation.The total excited-state lifetimes of Au144and Au246were determined to be around 3 and 4.6 ps respectively[32,55]. Since these two NCs have ultra-small energy gaps,the relaxation time from higher to lower excited-state will be comparable to the relaxation time from the lower excited-state to the ground state.Therefore,it is difficult to accumulate the excited-state population at the lower excited-state,leading to the disappearance of simple two-step relaxation in Au144and Au246(Figure 6c).
In the steady-state absorption,Au246exhibits a signif icant peak around 460 nm,which is similar to the SPR peak in metallic Au NPs.However,pump power independent excited-state dynamics and multiplepeaks in both steady-state and excited-state absorptions indicates that the giant 2.2 nm Au246should still be non-metallic[32].Moreover,one can observe signif icant oscillations superimposed on the electron dynamics of Au246.The frequency of the oscillation in Au246was determined to be around 16.7 cm-1(around 0.5 THz).Theseoscillationsin TA kineticsarisefrom thecoherent vibration of the metal core,which modif ies the absorption frequency and intensity periodically.They further conf irmed the metallicity of Au246NCs.Similar coherent oscillations can also be observed in the TA kinetics of Au130and Au144NCs[27].With the addition of only 33 gold atoms,Au279(SR)84(Au279for short)starts to show SPR absorption peak and power-dependent electron dynamics,which marks the emergence of metallic state[33].The optical properties of Au279and larger metallic Au NCs will not be further discussed in the review.
Asreported previously,if oneplot theexcited-state lifetime versus the energy gap of large sized Au NCs(n>100),the lifetime will decrease exponentially as the gap decreases(Figure 7a).According to the energy gap law,thenon-radiative decay rateknrcan be plotted as:[2]

whereEgrepresents the HOMO-LUMO gap,ωrepresents the highest vibrational frequency in the nonradiative decay,γrepresents the molecular parameter.Sincetheexcited-state relaxation of largesized Au NCs is dominated by non-radiative decay,knrcan be estimated by measuring the average TA lifetime.After plotting lnknrversusEg,one can observe that it has a linear relationship(Figure 7b).Therefore,by measuring the excited-state lifetime,we can estimate the energy gap of large sized gold nanoclusters from eq.(3).

FIG.7. (a)Excited-state lifetime versus energy gap for large sized Au NCs and(b)ln k nr versus energy gap.Reproduced with permission from ref.[2].Copyright 2021 American Chemical Society.
In general,the excited-state dynamics of large sized Au NCs are highly dependent on their size and energy gaps.Nevertheless,there are also some reports on the effect of structure on the photo dynamics of large sized Au NCs.Recent work reported the structure and electron dynamics of Au191(SR)66(Au191for short)NCs,which shows multiple absorption peaks but power-dependent electron dynamics(Figure 8a,b)[56].The face-centered cubic(fcc)kernel in Au191may play some role in modifying the excite state dynamics.In another work,the structure and optical properties of Au156(SR)60NCs were reported and it also showed molecular-like absorption peaks and power-dependent dynamics(Figure 8c,d)[57]. The uniquepower-dependent dynamics were ascribed to the anisotropic rod shape.It was reported that the rodshaped Au156should have more gold atoms on the long axis,which is longer than 2.2 nm and gives rise to power-dependent electron dynamics.These two examples indicated that the structure and shape should also betaken into consideration when considering themetallic versus molecular behaviors in large-sized(n>100)Au NCs.Based on the discussion,the excited-state relaxation diagram of metallic Au NPs and large sized Au NCs can be summarized in Figure 9.

FIG.8. (a)Global f itting results on the TA dynamics and(b)normalized principle kinetics of Au191 at different pump f luences.Reproduced with permission from ref.[56].Copyright 2020 American Chemical Society.(c)TA spectra at different time delays and(d)normalized TA kinetics of Au156(probed at 557 nm)at different pump f luences of Au156 NCs.Reproduced with permission from ref.[57].Copyright 2021 American Chemical Society.

FIG.9. Excited-state relaxation diagram of metallic Au NPs(left)and molecular Au n NCs(n>100)(right).
V.CONCLUSION AND OUTLOOK
In this minireview,I have discussed the electronic structure and optical properties of different sized Au nanoparticles,and the excited-state dynamics of large sized Au NCs(n>100).It is found that these large sized Au NCs bridge the molecular and metallic states in Au nanoparticles in terms of their excitedstate behaviors.
Here,I also provide some outlook on the future study of large sized Au NCs:First,the energy dissipation mechanism of large sized Au NCs calls for further research.The excited-state relaxation of Au NCs with ultrasmall energy gap may consist of both exciton relaxation and heat dissipation.A better physical model is required to understand the relaxation pathway.Second,the effect of structure and shaped on photo-physics remains to be investigated.Since there are only two sizes(Au156and Au191)found between Au144and Au246,more examples are required to further probe the effect on their dynamics other than size.Third,how doping and alloying affect the optical properties requires further study.Doping and alloying have shown a signif icant effect on the electronic structure and exciton dynamics of small-sized Au NCs.However,in large sized Au NCs,the effect of doping and alloying remains largely unknown.In general,by getting a deeper understanding of the energy dissipation mechanism in these large-sized NCs,we will be able to reveal the birth of SPR and the origin of metallic bonding in Au NPs,which will benef it their future applications.
ACKNOWLEDGMENTS
This work was supported by the startup funding from University of Science and Technology of China and the Chinese Academy of Sciences(Grant No.KY2340000137).
- 物理學(xué)進展的其它文章
- 非富勒烯受體有機光伏體系的激發(fā)態(tài)動力學(xué)
- 鈣鈦礦薄膜鈍化策略的研究進展