Structure and near-infrared luminescence of a dinuclear salen-type Nd(III) complex
ZOU Xiao-Yan, FEI Bo-Wen, LI Guang-Ming, *
(Heilongjiang University a. Editorial Board of Journal of Engineering of Heilongjiang University; b. Key Laboratory of Functional Inorganic Material Chemistry (MOE);c.School of Chemistry and Materials Science, Harbin 150080, China)
Abstract:A dinuclear lanthanide complex [Nd2L3(CH3OH)]·2CH3OH(1) supported by salen-type (H2L = N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine) ligand and Nd(OAc)3·H2O was structurally characterized. X-ray crystallographic analysis reveals that the two Nd(III) ions adopt same coordination environments that the octacoordinated Nd(III) ions are in a square antiprism geometry. Near-infrared (NIR) luminescent analysis reveals that complex 1 exhibit characteristic NIR luminescence of the Nd(III) ions enhanced energy transfer from the ligand to the Nd(III) ions in the solid state.The sale-type ligand can serve as an effeicient sensitizer for the characteristic luminescence of Nd(III) ions in CH3CN and CH3OH.The NIR luminescence intensity of complex 1 in CH3CN is stronger than that in CH3OH.
Key words:N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine; dinuclear; Nd(III) complex; coordination
Lanthanide complexes constructed from multidentate ligands are of considerable interest because of their unusual luminescent[1-4], and magnetic[5-10]. One of the well-known multidentate ligands, salen type, is able to stabilize different metals in various coordination environments. Previously, a number of studies have been focused on employing a variety of salen type ligands to stabilize lanthanide ions and provide the antenna for lanthanide luminescence[11-12].
Recently, attention has been also focused on complexes of Yb(III), Nd(III) and Er(III) with NIR emission in the range of 900 nm to 1 600 nm, which is highly transparent to biological systems and fibre media, because of their potential applications in bioassays and luminescent probes[13]. For example, Nd(III) ion for the basis of the common 1 064 nm laser and in silica-based fiber optic networks, closely match the “windows of transparency” in silica because of the increasing importance on utilization of NIR luminescent emission of Nd(III) ion in organic light-emitting diodes (OLED)[14]. In 2012, Weixu Feng et al. have presented a homoleptic tetranuclear [Nd4(L)2(HL)2(NO3)2(OH)2]·2(NO3) (H2L =N,N′-bis(salicylidene)cyclohexane-1,2-diamine) which shows strong and characteristic NIR luminescence of the Nd(III) ion[15]. In 2012, Pengfei Yan et al. reported two lanthanide complexes which are achieved by utilizing H2salen/acac ligands (H2salen =N,N-ethylenebis(salicylideneimine, acac = acetylacetonate). They are homonuclear [Nd4(μ3-OH)2(salen)2(acac)6(CH3OH)2]·CH3OH and heteronuclear [NdYb(salen)2(acac)2]. Homonuclear Nd4complex exhibit characteristic Nd(III) ions NIR emission. The heteronuclear NdYb shows ‘dual emission’ from both Nd(III) and Yb(III) ions[16].
The photophysical properties of Ln(III) ions depend on their ligand environments which can be designed to protect the metal center from solvent molecules which can quench emissions. Hererin, to further investigate the effect of salen-type lanthanide complexes on NIR luminescence properties as well as our long-standing research on this domain[17-19]and in view of the recent important progress on the structure, luminescence of salen-type lanthanide complexesN,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenyle-nediamine and lanthanide acetates were employed in the reactions to develop new salen type lanthanide complexes. As a result, the dinuclear complex Nd2L3(CH3OH)]·2CH3OH has been synthesized and NIR luminescence has been investigated.
1 Experimental Section
1.1 Materials and Instrumentation
All chemicals and solvents except Nd(OAc)3·H2O and H2L were obtained from commercial sources and used without further purification. The salen-type ligand(H2L) was prepared according to the literature and lanthanide precursors[20]. Elemental (C, H and N) analyses were performed on a Perkin-Elmer 2 400 analyzer. FT-IR data were collected on a Perkin-Elmer 100 spectrophotometer by using KBr disks in the range of 4 000~500 cm-1. UV spectra (in methanol) were recorded on a Perkin-Elmer 35 spectrophotometer. Thermal analyses were carried out on a STA-6000 with a heating rate of 10 ℃ min-1in a temperature range from 30 ℃ to 800 ℃ in atmosphere.
1.2 Crystallography
Single-crystal X-ray data of1was collected on a Rigaku R-AXIS RAPID imaging plate diffractometer with graphite-monochromated Mo Kα (λ = 0.071 07 nm) at 293 K. Empirical absorption corrections based on equivalent reflections were applied. The structure of1was solved by direct methods and refined by full-matrix least-squares methods onF2using SHELXS-97 crystallographic software package[21]. All non-hydrogen atoms are anisotropically refined. All crystal data and structure refinement details for complex1was summarized in Table 1.
1.3 Synthesis of complex 1
Synthesis of [Nd2L3(CH3OH)]·2CH3OH (1). A solution of Nd(OAc)3(0.4 mmol) in CH3OH (10 mL) was added to a solution of H2L (0.2 mmol). The mixture was stirred under ambient temperature for 12 h. The color of the mixture turned from orange to luminous yellow. Then, diethyl ether was diffused slowly into the solution. Yellow crystals of1were obtained.
C73H67N9O13Nd2(1 565.48) (1) Yield: 0.123 3 g (62.7%). Elemental analysis (%) calcd for C, 55.95; H, 4.28; N, 8.04 %. Found: C, 55.80; H, 4.37; N, 8.16 %; IR (KBr, cm-1): 3 414(m), 1 615(s), 1 472(m), 1 246(s), 1 196(m), 741(m). UV-vis [MeOH, λ]: 239, 298, 386 nm.
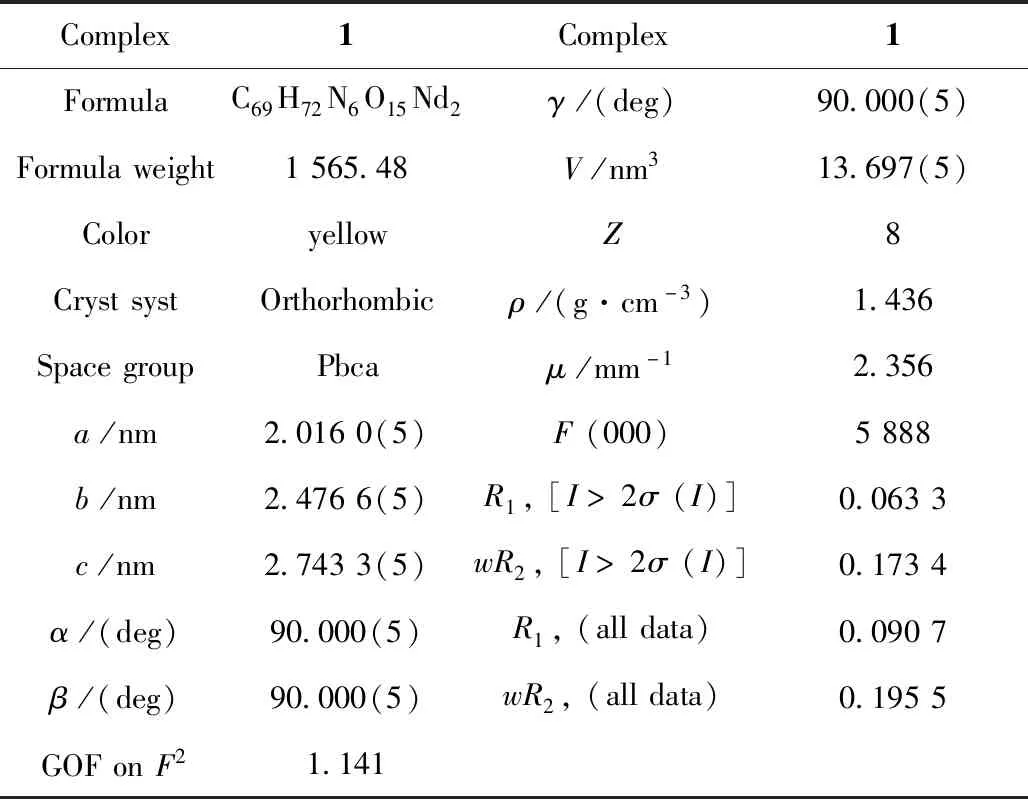
Table 1 Crystal data and structures refinement for complex 1
2 Results and discussion
2.1 Spectral analysis
Infrared spectra of the ligand and complex1is showed in Fig.1a, In a typical spectrum of1, the broad weak O-H stretching vibration at 3 414 cm-1disappeared, while a weak and broad band at about 3 421 cm-1is newly generated from the N-H vibration. The strong v (C=N) bands occurring in the range of 1 648~1 654 cm-1for complex1shifts to higher wavenumber in comparison with that for free H2L (1 636 cm-1), due to the coordination of ionized OH groups, which reduces the strengthening of C=N groups.
The UV-Vis spectra of the ligand and complex1are recorded in MeOH solution (Fig.1b). For ligand, the typical absorptions at 215, 240 and 309 nm are attributed to the π-π* transition of the aromatic ring and azomethine chromophore. For complex1, the similar ligand-centered solution absorption bands (230, 263, 336 nm) are observed and red-shifted as compared to those (215, 240 and 309 nm) for ligand resulting from the changes in the energy levels of the ligand orbitals upon the coordination of the Nd(III) ion.
2.2 TG-DSC analysis
TG-DSC analysis of complex1is showed in Fig.2. In a typical curve of complex1exhibits a gradual weight loss of 4.30% in the range of 120~300 ℃, which corresponds to the loss of two CH3OH molecules (calcd 4.35%). TG-DSC data further confirm that two CH3OH molecules exist in complex1.

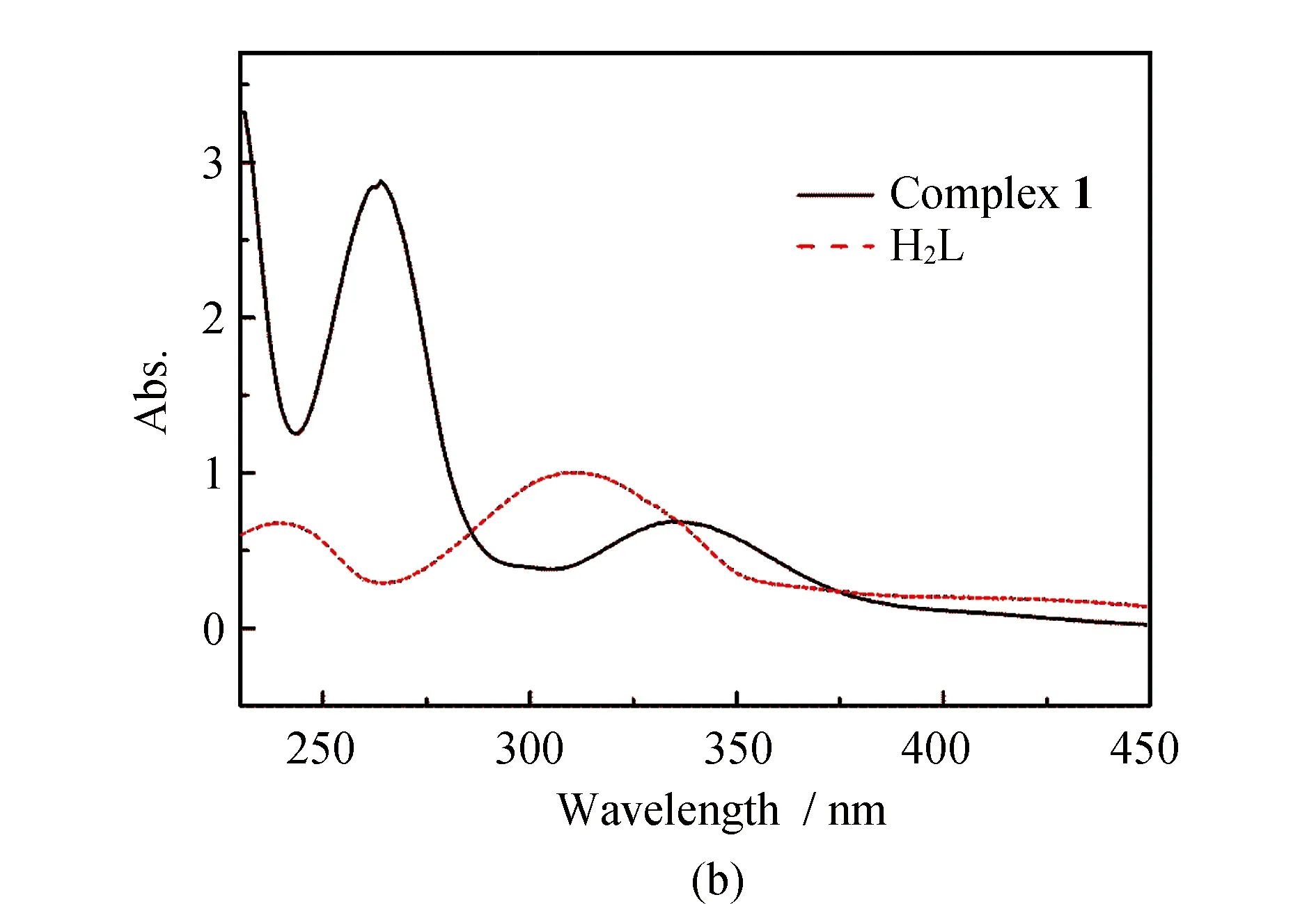
Fig.1 IR spectra of the H2L and complex 1 (a); UV absorption spectra of the H2L and complex 1 (b)
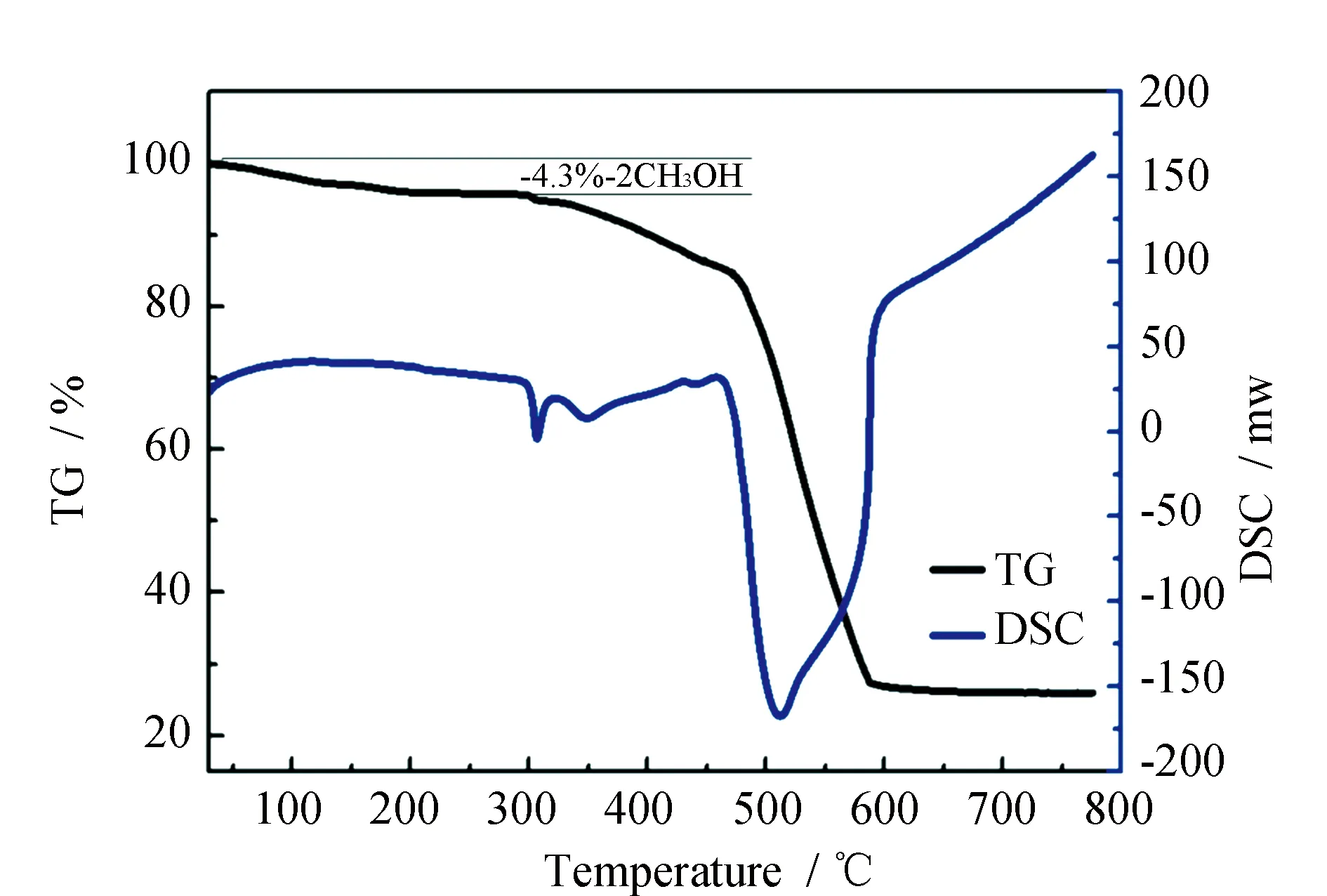
Fig.2 TG-DSC curves of complex 1
2.3 PXRD analysis
Powder X-ray diffraction (PXRD) patterns of complex1is in agreement with the simulated ones (Fig.3). PXRD analysis further demonstrates that the crystal structure of complex1is truly representative of the bulk materials. The differences in intensity are due to the preferred orientation of the powder samples.

Fig.3 The powder X-ray diffraction patterns and the simulated patterns of complex 1
2.4 Structural description of complex 1
X-ray crystallographic analysis reveals that complex1crystallizes in an orthorhombic space group of Pbca. As shown in Fig.4a, complex1contains three ligands, two Nd(III) ions, and one from two methanol molecule, respectively, forming a bicappedtrigonal prism geometry with C2vpoint group (Fig.4b). The two Nd(III) ions are bridged by thephenolate O1, O3, and O6 atoms with a distance of 0.388 58 nm. The Nd-O bond lengths are in the range of 0.218 3~0.283 1 nm and the Nd-Nd bond lengths are 0.246 2 to 0.254 4 nm respectively, which is longer than previously reported [Nd2(L8)2(OAc)2(MeOH)2]n(H2L8=N,N′-ethylene bis(salicylideneimine))(Fig.4b). Although both complex1and [Nd2(L8)2(OAc)2(MeOH)2]nare alike in terms of the compositions and dinuclear structure, they are distinguished from the ratio of H2L/Nd. For example, the ratio of H2L/Nd is 2∶2 for complex [Nd2(L8)2(OAc)2(MeOH)2]n, while the ratio of H2L/Nd is 1∶2 for complex1in which the two Nd(III) ions are in the cavities of N2O2 and O2O2 of one ligand, respectively. The adjacent binuclear molecules are packed through the π-π interactions (0.265 32(2)-0.248 36(4))(Fig.5).
2.5 NIR Luminescence
The NIR luminescence of the complex1has been investigated in solid, CH3CN and CH3OH. The emission spectrum of complex1(Fig.6) presents three bands at 896, 1 060, and 1 312 nm, which can be assigned to4F3/2→4I9/2,4F3/2→4I11/2and4F3/2→4I13/2transitions of the Nd(III) ion. The lifetime cannot be obtained due to the weakness of the signal and the limitations of our equipment. In addition, The results reveal again the enhanced energy transfer from the ligand to the Nd(III) ion in the solid state compared to that in solution. The NIR luminescence intensity of complex1in CH3CN is stronger than that in CH3OH. This is attributed to the O-H oscillators in CH3OH, coupling of overtones and the excited state of Nd(III) ion, resulting in the nonradiative transition, which quench the NIR luminescence.

Fig.4 Cationic structure of complex 1(a) and the coordination geometries of Nd1(III) and Nd2(III) ions(b)
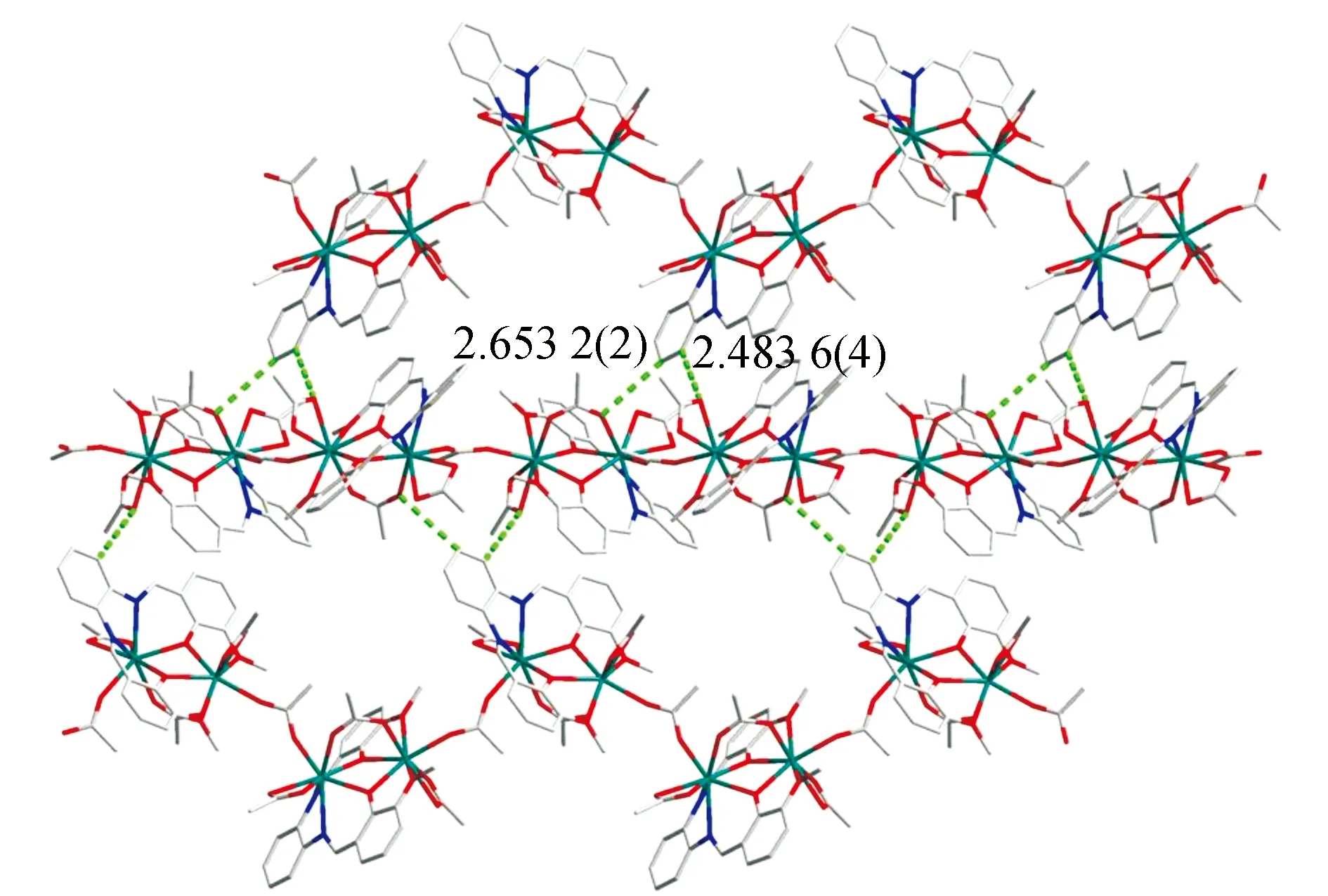
Fig.5 2D supramolecular network of 1 formed by the intermolecular hydrogen bonds
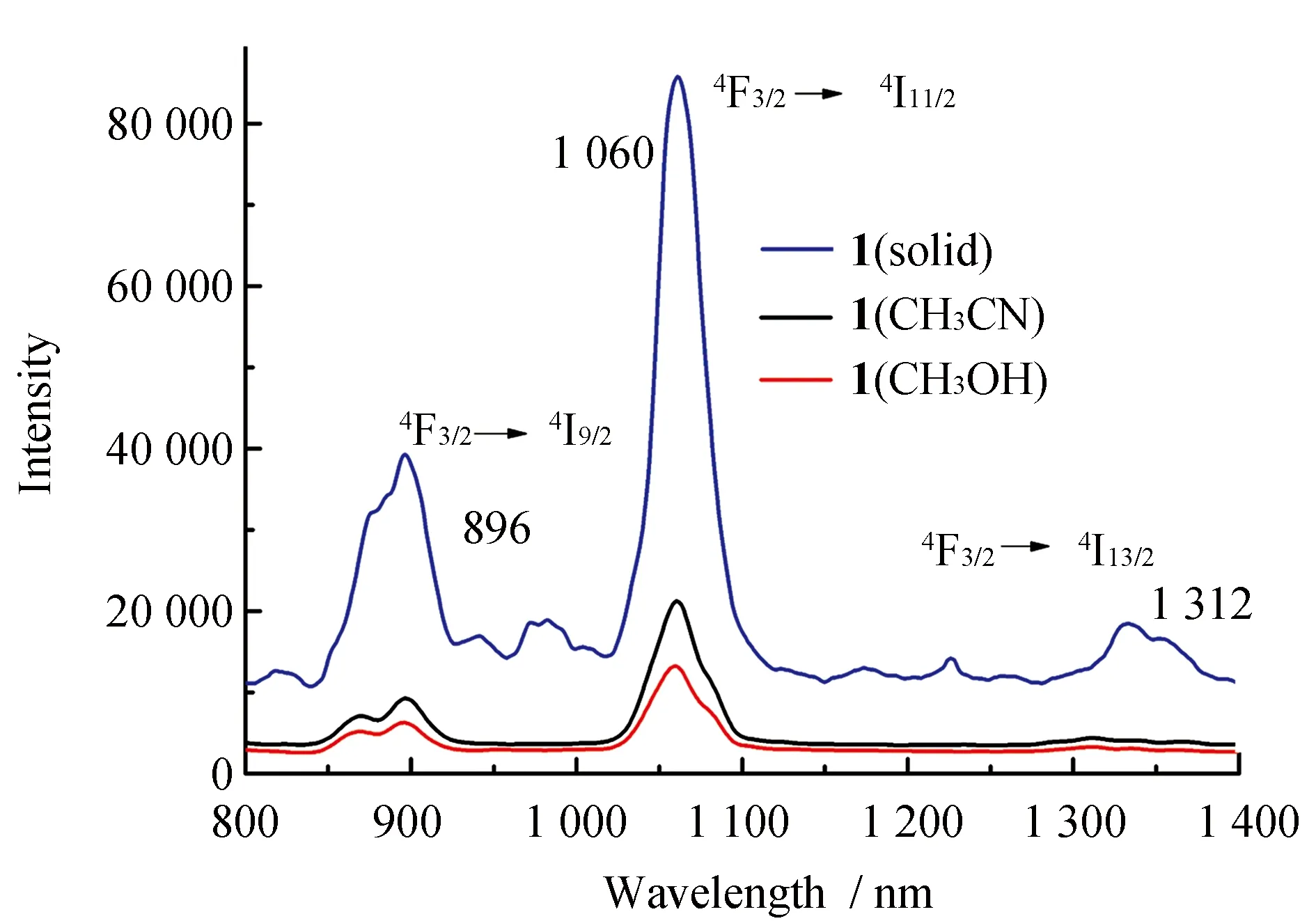
Fig.6 NIR luminescence spectra of 1 in solid, CH3CN and CH3OH (1.0×10-5 M)
3 Conclusions
Isolation of complex1demonstrates that synthesis of salen type dinuclear complex with rigid salen-type (H2L=N,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine) ligand are possible, and the structure of the salen type ligand dominate the structure of the complex and the coordination geometries of the Nd(III) ions. Complex1is analogous possessing two unique lanthanide ions with same coordination geometries according to the microanalysis, TG-DSC and crystal analysis. NIR luminescence of complex1suggests that theN,N′-bis(2-oxy-3-methoxybenzylidene)-1,2-phenyl-enediamine can serve as an efficient sensitizer for the characteristic luminescence of Nd(III) ions.

