Synthesis and electrochemical properties of Zn3V3O8 as novel anode material
Yunxi Jin,Junjie He,Zihu Ou,Chunqi Feng,*,Gungxue Zhng**
a Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials & Ministry of Education Key Laboratory for Synthesis and Applications of Organic Functional Molecules,Hubei University,Wuhan 430062,China
b School of Nuclear Technology and Chemistry & Biology,Hubei University of Science and Technology,Xianning 437100,China
c Nanxian Fanggu Middle School,Yiyang 413211,China
Keywords:
ABSTRACT
Zn3V3O8 two-dimensional micro sheets are successfully synthesized by combination of solvothermal method and heat treatment.The X-ray diffraction(XRD)and field emission scanning electron microscopy(SEM)are used to characterize the structure and morphology of the samples,and the battery testing system is used to investigate its electrochemical performance.The results show that the Zn3V3O8 is successfully obtained using mix solvent(water:ethylene glycol=1:1),and the sample takes on morphology of two-dimensional sheets.The initial discharge capacity for Zn3V3O8 two-dimensional micro sheets is 752.0 mAh/g at 100 mA/g in the voltage range of 0.01-3 V.After heat treatment in a tube furnace at 550°C,the sample had the initial discharge capacity as 1152.0 mAh/g and maintained it at 901.4 mAh/g after 100 cycles.The good electrochemical performance for Zn3V3O8 two-dimensional micro sheets make it possible to be used as novel anode for lithium ion battery application.
In recent years,lithium ion batteries(LIBs)with high specific energy have been widely applied in the hybrid electric vehicles and power supplies of electric[1].It is high time to find new electrode materials with high capacity and excellent cycle performance.Transition metal vanadates attract researchers'attentions because of its special microstructure,high theoretical capacities and good safety.Therefore,vanadates with different morphologies and structures have been widely studied as anode materials for lithium-ion batteries in recent years [2-17].And the special microstructure of vanadates will reduce the diffusion path of lithium ions in the electrode and behave good electrochemical performance.For example,P.Liu studied the application of vanadium-based oxides with 3D micro/nano-structures on energy storage[10].Nevertheless,many vanadium oxides are needed to be studied further.
In this work,mix-solvothermal method is used to synthesize precursor firstly.Then Zn3V3O8two-dimensional micro sheets are obtained by sintering the precursor under Ar atmosphere at 350°C for 2 h.The electrochemical properties for Zn3V3O8sample as anode material were studied also.The results are meaningful to develop new anode material.
The reaction solvent in solvothermal was prepared by mixing water and ethylene glycol with volume ratio of 1:1.Firstly,2 mmol NH4VO3was dissolved in 30 mL the mixed solvent,and then 2 mmol Zn(NO3)2·6H2O was added into the solution.After magnetic stirring for about 30 min,the reactants were completely dissolved.Afterwards,the solution was transferred to autoclave and heated at 200°C for 18 h.After being cooled down to room temperature,the result samples were collected by washed with deionized water and ethanol several times.Finally,the precursor was dried at 60°C in a vacuum oven for 12 h.The obtained precursor was labeled as sample-1.The precursor(sample-1)was sintered under Argon atmosphere at 350°C for 2 h to obtain final product(Zn3V3O8)which was labeled as sample-2.
Some analytical methods have been developed to measure the samples.Fig.1 shows the XRD patterns of the samples synthesized before and after calcinations.As shown in Fig.1,the XRD patterns of the two samples are consistent with the stand card of Zn3V3O8(JCPDS File Card No.31-1477).There are some hybrid peaks in the XRD pattern of sample-1,which indicates that the sample-1 is not purity.However,there are no other impure peaks to be detected in sample-2,indicating that pure Zn3V3O8was formed after calcinations.Besides,the intensity of diffraction peaks of sample-2 is stronger than that of sample-1,which proved that Zn3V3O8had good crystallinity.
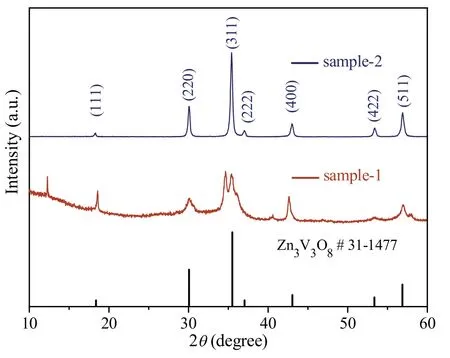
Fig.1.XRD pattern of the Zn3V3O8 samples synthesized before and after calcinations.
Then,the microcosmic morphologies of the precursor(sample-1)and final product(sample-2)were detected by SEM techniques.Fig.S1(Supporting information)shows the SEM images of sample-1 and sample-2.The SEM images of sample-1 are shown in Figs.S1a and b,and it was seen that precursor takes on morphology of micro sphere which is assembled from two-dimensional sheets.However,sample-2 in Fig.S1 are several two-dimensional sheets,which proved that micro sphere were collapsed to become regular twodimensional sheets after its sintering at 350°C for 2 h.
Inaddition,theelectrochemicalperformancesof thesampleswere tested by battery test system.Fig.2 and Fig.S2(Supporting information)present the typical charge/discharge curves of the sample-1 and sample-2 at a current density of 1000 mA/g and 100 mA/g.Fig.S2a shows that the initial discharge and charge capacities of sample-1 are 483.9 mAh/g and 752.0 mAh/g and its discharge capacities can maintain at 225.0 mAh/g after 60 cycles.The columbic efficient in the first cycle is about 64.35%,indicating that there is serious irreversible capacity loss during the first discharge process.
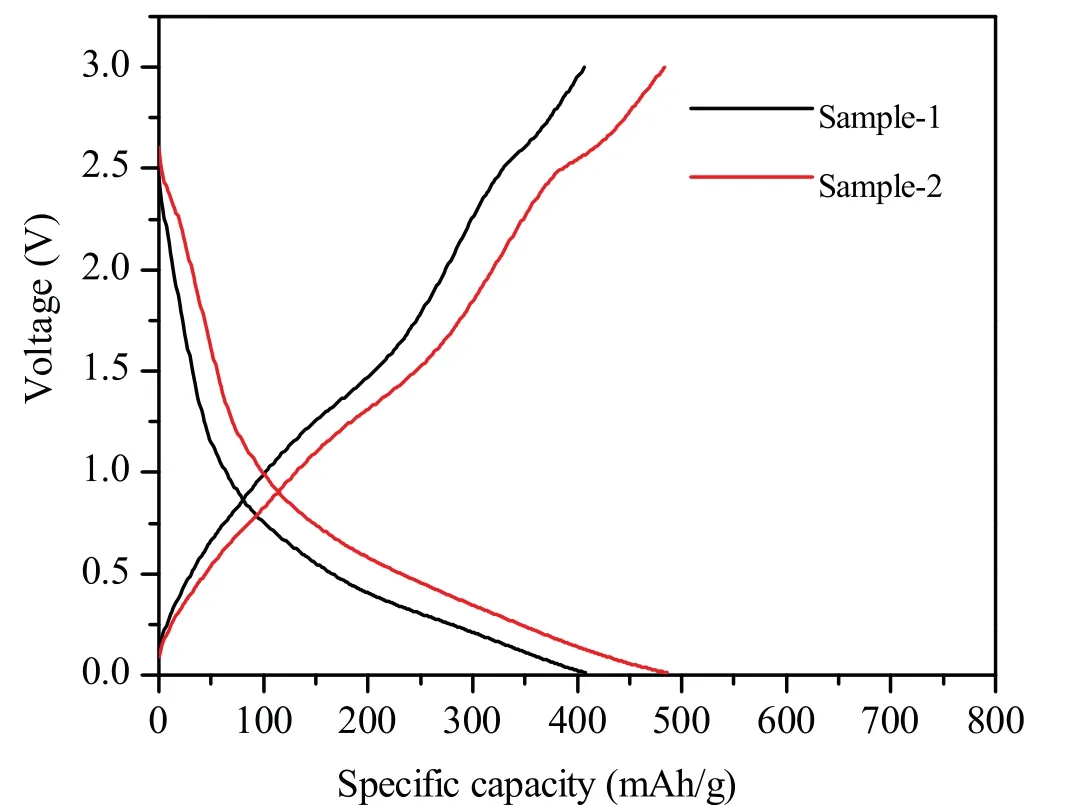
Fig.2.Typical charge/discharge curves of sample-1 and sample-2 at a current density of 1000 mA/g.
The electrolyte decomposition and formation of a solid electrolyte interphase(SEI)filmonthesurfaceoftheanodematerialcancausethe large irreversible capacity loss in the initial cycle.As shown in Fig.S2b,the initial discharge and charge capacities of sample-2 are 1152.0 and 914.2 mAh/g,andcolumbicefficientisabout79.36%.Fig.3presentsthe typicalcharge/dischargecurvesofsample-1andsample-2atacurrent densityof1000 mA/g,thedischargeandchargecapacitiesofsample-2 are486.32and483.25 mAh/g,whicharebetterthansample-1(408.56 and 406.71 mAh/g).The columbic efficient of sample-2 in the 100th cycle is about 99.37%.Fig.3 presents the cycling at a current density of 1000 mA/g(Fig.3a)and rate performance for sample-1 and sample-2 at a current density of 100 mA/g(Fig.3b).As shown in Fig.S3(Supporting information),sample-2 presents better cycle performance than that of sample-1.After 100 cycles at the 100 mA/g current densityinthevoltagerangeof0.01-3.0 V,thesample-2anodeexhibits a high capacity of 901.4mAh/g while the sample-1 just has capacity of 225.0 mA/g.Moreover,the columbic efficient of sample-2 remain at over 99%.Interestingly,the discharge capacity of sample-1 first decreasesduringfirst20cycles,thengraduallyincreasesinthenext80 cycles.And the discharge capacity of sample-1 also has the similar phenomenon.Thedecrementofdischargecapacityattheinitialcycles canbeascribedtotheformationofSEIlayer[12].Andthelaterincrease ofdischargecapacitycanbeattributedtotheprogressiveinteractionof lithium-ions in electrolyte solution with Zn3V3O8nanosheets from surface to inside [13],and normally attributes to the presence of a possible activation process in the electrode[14].The appearance has also been observed in many other transition mental oxide anodes[2,12-14].
The cycling stability of sample-1 and sample-2 at a current density of 1000 mA/g over 100 cycles was illustrated in Fig.3a.The sample-2 delivers an initial discharge capacity of 723.28 mAh/g and still maintained a remarkable capacity of 483.25 mA h/g after 100 cycles.By comparison,the sample-1 delivers an initial discharge capacity of 681.16 mAh/g and maintained a remarkable capacity of 406.71 mAh/g after 100 cycles.The results show that the sample-2 has better electrochemical performance.
Fig.3b presents the rate capability at current densities of 100,200,400,800,1000 and 2000 mA/g.The corresponding discharge capacities for the sample-2 are 954.2,870.5,800.4,756.2,692.3 and 612.5 mAh/g.When the current density is reduced to 100 mA/g,the discharge specific capacity recovers to 942.3 mAh/g.Compared with sample-1,it shows better rate performance.From the above electrochemical properties,it is clearly seen that sample-2 not only has higher specific capacity but also better cycle performance than thatofsample-1.Itcanbeattributedtothemorecompeteandregular two-dimensional sheet,which has shorter distance for lithium ions tobeinsertedandcomeout.Incomparisonwithotherwork recently,this result in our study is very satisfied
EIS measurements were conducted to further clarify the electrochemical performances.Fig.S4(Supporting information)presents the nyquist impedance plots of the sample-1 and sample-2.It is easily found that sample-2 have a lower charge-discharge resistance(Rct)than sample-1.This proves that the sample-2 have better chargetransfer performance and electronic conductivity,which should be attributed to the regular two-dimensional sheet.
In conclusion,the Zn3V3O8was successfully synthesized by combination of solvothermal method and heat treatment when mixed solvents(water:ethylene glycol=1:1)were used.The electrochemical properties for sample-1 is not as satisfied as we expected.After sintering at 350°C for 2 h,the sample-2 has more complete frame structure and behaves better cycle and rate performance.Its reversible capacity is 901.4 mA/g after 100 cycles,which is much better than that of sample-1.The superior electrochemical properties show that heat treatment is beneficial to synthesize Zn3V3O8with morphology of two-dimensional sheets and high purity.Therefore,Zn3V3O8behaved excellent electrochemical properties as anode material.The Zn3V3O8synthesized by this method is a promising anode material for lithium ion battery application.
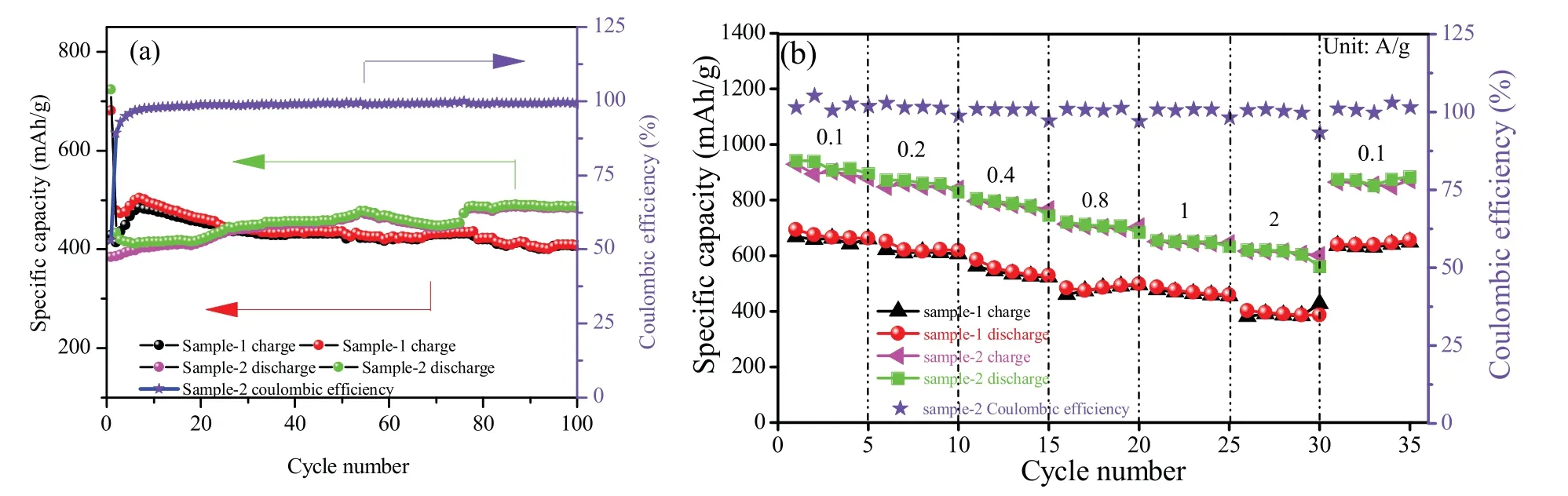
Fig.3.The cycling at a current density of 1000 mA/g(a)and rate performance for sample-1 and sample-2 at a current density of 100 mA/g(b).
Acknowledgment
Financial support by the National Natural Science Foundation of China(No.21476063)is gratefully acknowledged.
Appendix A.Supplementary data
SSupplementary material related to this article can be found,in the on line version,at doi:https://doi.org/10.1016/j.cclet.2018.12.010.
 Chinese Chemical Letters2019年3期
Chinese Chemical Letters2019年3期
- Chinese Chemical Letters的其它文章
- Ligand controlled structure of cadmium(II)metal-organic frameworks for fluorescence sensing of Fe3+ ion and nitroaromatic compounds
- Large-scale synthesis of size-controllable Ag nanoparticles by reducing silver halide colloids with different sizes
- Improved electrochemical properties in the Li3Fe2(PO4)3 by titanium and vanadium doping
- Preparation of nitrogen doped clews-like carbon materials and their application as the electrode in supercapacitor
- Preparation and optical properties of three-dimensional navel-like Bi2WO6 hierarchical microspheres
- Bioresponsive nanogated ensemble based on structure-switchable aptamer directed assembly and disassembly of gold nanoparticles from mesoporous silica supports
