Bioresponsive nanogated ensemble based on structure-switchable aptamer directed assembly and disassembly of gold nanoparticles from mesoporous silica supports
Zhiyong Zhang*,A.Runa,Jie Wu,Han Zhang,Xia Li,Zhiqiang He
Inner Mongolia Power Research Institute,Hohhot 010020,China
Keywords:
ABSTRACT
By taking advantage of recent advances in aptamer biology and nanotechnology,we developed a general approach for the design and fabrication of bioresponsive controlled delivery systems.It utilized the structure-switchable aptamer directed assembly and disassembly of gold nanoparticles from mesoporous silica supports,which enables the control of cargo release from the inside of the mesoporous nanoparticles specifically in the presence of target molecule.
The mesoporous silica nanoparticle(MSN)based nanocarriers has received increasing attentions due to their outstanding structural characteristics and promising application in controlled drug delivery [1-5].To date,various MSN-based smart nanocarriers have been constructed that contain different kinds of gatekeepers such as inorganic nanoparticles,organic molecules,and supramolecular assemblies that respond to a range of physicochemical stimuli such as pH [6-12],temperature [13],redox potential [14-18],photoirradiation [19-21],or enzyme[22,23] in order to trigger the release of the loaded cargo molecules.However,despite some recently reported gated MSNs that could be uncapped by certain enzymes [24-26],antibodies[27] or carbohydrates [28],the development of MSN-based nanocarriers with autonomously controlled nanovalve opening by specific native biomolecules is still a big challenge in this field.
The sequence-specific interactions of DNA and its defined structure and conformation in solution and unique programmable nature to tune its functional properties make it a powerful biopolymer for chemical and biochemical applications [29-31].Most recently,several controlled release systems have been reported through coupling DNA with MSN materials,but their stimuli were limited to temperature [32],target DNA [33] or endonucleases[34].On the other hand,functional DNA is DNA with functions beyond genetic information storage and possesses properties similar to those of proteins,including catalysis(called DNAzymes),specific ligand binding(called aptamers),or a combination of both(called aptazymes)[29-31].In particular,aptamers are single-stranded functional DNA molecules that can bind a wide array of biological targets with high affinity and with specificity comparable to that of antibodies,including small molecules,proteins,and even viruses and cancer cells [23-31,35,36].Furthermore,aptamers offer distinct advantages over antibodies such as small size,easy production without the need of an animal source,and good stability against biodegradation and denaturation,which make them promising molecular receptors for diagnostic and therapeutic applications [29-31].Recently,Yang and co-workers have used ATP aptamer to construct MSN-based controlled release system[37].However,the replacement mechanism they used make the method complex and difficult to apply for other bio-targets.Herein,we introduce structure-switchable aptamer into MSN-based nanocontainers and present a general method for the design of biomolecule responsive on demand delivery system.
The structure-switching idea takes advantage of the universal ability of any aptamer to switch between a duplex structure and an aptamer-target complex,which has been widely used to construct various biosensors [38-42].Fig.1 shows how we combined the significant characteristics of structure-switchable aptamer and mesoporous silica to construct a simple,but efficient nanogated ensemble,where adenosine aptamer was chosen to demonstrate adnoine-triggered controlled release of cargo molecules from MSNs.The DNA1 attached to gold nanoparticles(AuNPs)at its 5ˊ end is predesigned by introducing the adenosine aptamer fragment(in green)and a pentanucleotide sequence(in gray).The DNA2 is grafted on the external surface of MSN at its 3ˊend and is complementary to the pentanucleotide and to the first portion of the adenosine aptamer.In the presence of target,the aptamer DNA(in green)switches its structure and binds two adenosine molecules [38-43].As a result,only the pentanucleotide(five DNA base pairs)is left to bind MSN,which is unstable and thus disassembled the AuNPs from the surface of MSN,with an accompanying cargo molecules release from the inside of the MSN.
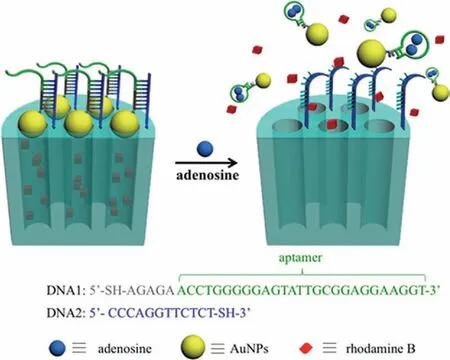
Fig.1.Schematic illustration of bioresponsive nanogated ensemble based on structure-switchable aptamer directed assembly and disassembly of gold nanoparticles from mesoporous silica supports.
MSNs were synthesized by following a base-catalyzed sol-gel procedure reported by Lin and co-workers [14,15].The resulting silica nanoparticles(ca.100 nm)that contain MCM-41-type channel-like mesoporous structure was confirmed by TEM and X-ray diffraction(Figs.S1 and S2 in Supporting information).An average pore diameter of 2 nm was estimated from the TEM image,which compares well with the literature report[14].The external surface of MSN was functionalized with amine groups by treatment with 3-aminopropyltriethoxysilane(APTS),followed by the removal of the surfactant template to give MSN-NH2.The N2adsorption-desorption isotherms of MSN-NH2further revealed a type IV curve with a specific surface area of 850 m2/g(Fig.S3 in Supporting information).
In order to attach DNA on the MSNs,we first prepared the maleimide terminated MSN(MSN-Mal)by reacting MSN-NH2with the bifunctional cross-linker sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate(Sulfo-SMCC)which contains an amine-reactive N-hydroxysuccinimide(NHS ester)and a sulfhydryl-reactive maleimide group(Scheme S1 in Supporting information).The resulted maleimide terminated silica particles were subsequently conjugated with the 3ˊ-thiol-modified DNA2.The immobilization efficiency of DNA2 on the MSN surface was determined to be 5.2μmol/g SiO2based on UV/vis spectroscopy.The efficient attachment of DNA2 onto MSN was also validated by the appearance of the characteristic asymmetric stretching mode of the imidyl group(1710 cm-1)and the typical amide vibrations(1550 cm-1),in the FTIR spectroscopy(Fig.S5 in Supporting information).
DNA functionalized AuNPs were used to cap the MSNs.5ˊ-Thiolmodified DNA1 were chemically attached to the AuNPs(5 nm)according to our previously reported method [15].The DNA2 immobilized MSN was incubated with the DNA1 functionalized AuNPs in the hybridization buffer to form the DNA duplexes and thus to cap the gold nanoparticles onto the mesoporous silica(MSN-apt-AuNPs).The successful capping and uncapping was directly confirmed by TEM microscopy.Fig.2 showed the TEM micrographs of MSN-apt-AuNPs before and after addition of adenosine molecule.In the case of the capped MSN(Fig.2a),dark spots on the outside of the mesopores were clearly observed,representing the assembly of AuNPs on the exterior surface of MSN.In contrast,by addition of adenosine molecule,the disassembly of AuNPs from MSN was observed in Fig.2b.These results indicated that the target analyte induced structure switch of the aptamer indeed uncap AuNPs from the MSN.
To investigate the target-triggered controlled release property of the hybrid ensemble,rhodamine B that used as a model guest molecule was loaded into the pores of silica particles by soaking MSN-DNA2 in a solution of rhodamine B.Capping the pores was achieved by adding the DNA1-functionalized AuNPs into the mixture for a DNA-mediated self-assembly.After incubation overnight,the excessive dye was removed by centrifugation and repeated washing with Tris-acetate buffer(20 mmol/L Tris-acetate,300 mmol/L NaCl,0.05%Tween-20,pH 8.2).The resulting particles were redispersed in the Tris-acetate buffer,and the uncapping and subsequent delivery of the dye was monitored through the measurement of the absorbance maximum of rhodamine B(553 nm)in solution as a function of time.As shown in Fig.3A,the nanogated ensemble exhibited a negligible leakage of the entrapped dye molecules in the absence of target,thus indicating a good end-capping efficiency.In contrast,the introduction of 5.0 mmol/L adenosine resulted in a significant release of the dye molecules,which was attributed to the disassembly of AuNPs from MSN mediated by the target induced structure switching of aptamer.The controlled release behavior with a series concentration of adenosine has also been investigated(Fig.3B).It is apparent that the dye release is positively dependent on the target concentration,thus demonstrating that the delivery system reported here thus could be quantitatively mediated.The most important element in designing such nanogated ensemble is the number of base pairs of the pentanucleotide sequence of the DNA1 on AuNPs,and the number of nucleotides from the aptamer sequence that are involved in binding to MSN.The binding should be strong enough to hold the AuNPs to cap the pores but still allow the aptamer to bind target molecules and undergo rapid structure switching.
We further tested the specificity of aptamer-based nanovalve for adenosine.In Fig.4,in the absence of adenosine or in the presence of 5 mmol/L other nucleoside analogues such as cytidine,uridine or guanosine,the MSN-apt-AuNPs did not show a characteristic release.These results clearly demonstrate the operation of our nanovalve could only be triggered by the specific target molecule,without the interference of other nucleoside,which is ascribed to the high binding affinity and specificity of aptamer.
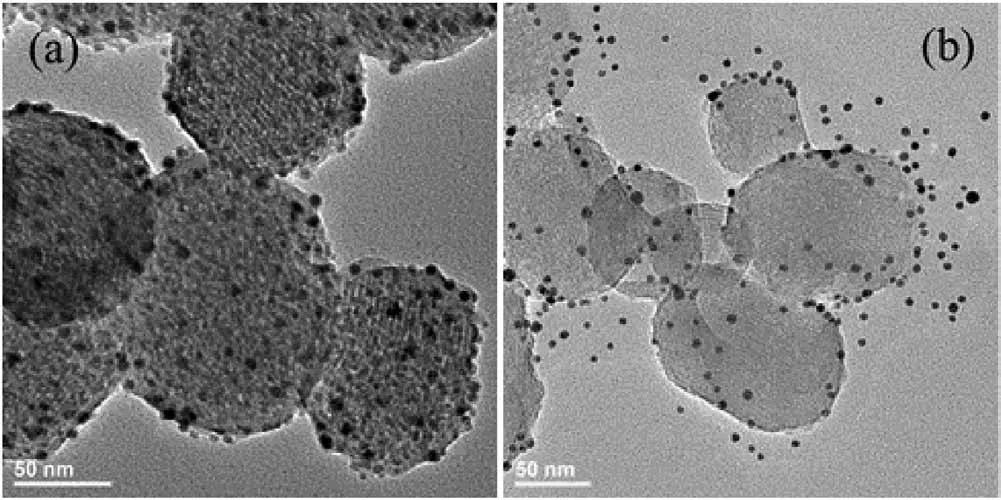
Fig.2.TEM micrograph of the nanogated ensemble MSN-apt-AuNPs before(a)and after(b)the reaction with target adenosine molecules.
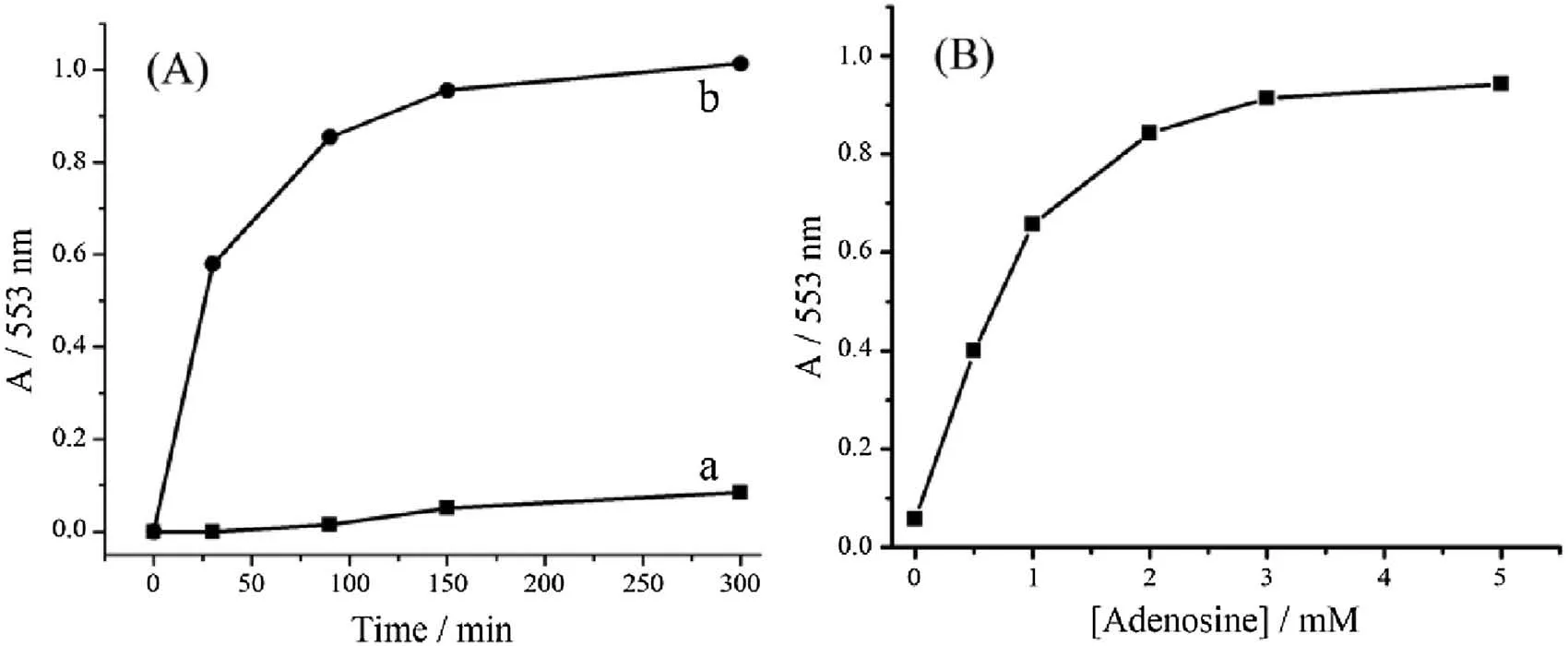
Fig.3.(A)Controlled release profile of rhodamine B loaded MSN-apt-AuNPs(a)in the absence and(b)in the presence of target adenosine molecules(5 mmol/L);(B)The controlled releases plotted as the function of adenosine concentration,measured after 150 min of the target addition.
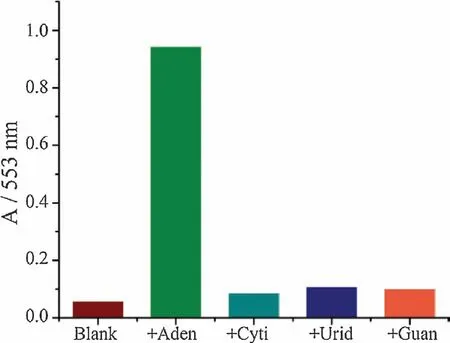
Fig.4.The release selectivity of rhodamine B loaded MSN-apt-AuNPs toward different nucleoside analogues:adenosine,cytidine,uridine,and guanosine,measured after 150 min of the analyste addition.
In conclusion,we employed structure-switching behavior of aptamer to demonstrate a general paradigm for the design and fabrication of bioresponsive controlled delivery system based on the assembly and disassembly of gold nanoparticles from mesoporous silica supports.The guest molecules trapped inside the nanopores and blocked by AuNPs can be selectively released in the presence of target adenosine molecules.The adenosine aptamer-based design serves as a model system for similar“smart” bioresponsive nanogated MSN systems.While demonstrably powerful,the design of such bioresponsive nanogated ensemble is restricted by structure-switching requirements of aptamer.Since aptamers specific for a variety of molecular markers and metabolites in the biological systems can be obtained through SELEX,this method can be applied to obtain delivery systems able to be selectively triggered by a diverse range of bio-targets.
Acknowledgment
This work was supported by the Inner Mongolia Power(Group)Co.,Ltd.,Technology Project(No.2016-20).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2018.10.019.
 Chinese Chemical Letters2019年3期
Chinese Chemical Letters2019年3期
- Chinese Chemical Letters的其它文章
- Synthesis and electrochemical properties of Zn3V3O8 as novel anode material
- Ligand controlled structure of cadmium(II)metal-organic frameworks for fluorescence sensing of Fe3+ ion and nitroaromatic compounds
- Large-scale synthesis of size-controllable Ag nanoparticles by reducing silver halide colloids with different sizes
- Improved electrochemical properties in the Li3Fe2(PO4)3 by titanium and vanadium doping
- Preparation of nitrogen doped clews-like carbon materials and their application as the electrode in supercapacitor
- Preparation and optical properties of three-dimensional navel-like Bi2WO6 hierarchical microspheres
