Synthesis and Crystal Structure of a New Complex [Cu(C14H9O3)2(C5H5N)2(C2H5OH)2]①
HU Wei-Bing SONG Nan-Nan HUANG Meng FENG Fu TIAN Da-Ting ZHOU Hong-Yan SONG Xin-Jian
?
Synthesis and Crystal Structure of a New Complex [Cu(C14H9O3)2(C5H5N)2(C2H5OH)2]①
HU Wei-Bing②SONG Nan-Nan HUANG Meng FENG Fu TIAN Da-Ting ZHOU Hong-Yan SONG Xin-Jian
(445000)
The title compound of Cu(C14H9O3)2(C5H5N)2(C2H5OH)2(1) was synthesized viathe hy- drothermalreaction of CuCl2·2H2O and 9-hydroxy-fluorene-9-carboxylic acid (HHF) with pyridine, andcharacterized by elemental analysis and infrared spectra. The crystal belongs to triclinic,space group1 with= 8.8302(12),= 10.1625(14),= 12.2708(17) ?,= 86.207(2),= 69.562(2),= 64.932(2)o,=930.3(2) ?3,= 1,M= 764.30,D= 1.364 g/cm3,(000) = 399,= 1.059 and(Mo) = 0.644 mm-1. The final= 0.0459 and= 0.1274 for 3414 observed reflections with> 2(). The copper atom is six-coordinated by two oxygen atoms from two different 9-hydroxy-fluorene-9-carboxylate ligands, two pyridine nitrogen atoms and two ethanol oxygen atoms, forming a distorted octahedral coordination geometry.The extensive O–H···O hydrogen bonding connects the molecules to form a one-dimensional chain structure. Between adjacent one-dimensional chains, a two-dimensional layered structure was formed by fluorene ringpacking interaction. Between the layers, a three-dimensional structure was formed through thepacking interaction of the pyridine ring. Moreover, the thermal stability and photoluminescent property of the complexhas been investigated.
9-hydroxy-fluorene-9-carboxylic acid, synthesis, crystal structure
1 INTRODUCTION
Fluorene and its derivatives are very useful com- pounds due to their good optical property and high luminescent efficiencies, and have received a lot of attention[1-4]. Two hydrogen atoms in fluorene 9 digit are replaced by hydroxyl and carboxyl that can generate9-hydroxy-fluorene-9-carboxylic acid. It has biological activity,and can delay the plant life and influence plant root gravitropism and stem phototropism[5].Moreover,the 9-hydroxy-fluorene- 9-carboxylic acid contain two different coordination sites of hydroxyl and carboxyl, which were good potential building complex organization. At present, relevant synthesis and structure chemical research of it and metal ion are relatively less. In this paper, we synthesize a novel compound of Cu(C14H9O3)2- (C5H5N)2(C2H5OH)2viathe hydro- thermalreaction of CuCl2·2H2O and 9-hydroxyfluorene-9-carboxylic acid (HHF) with pyridine.
2 EXPERIMENTAL
2. 1 Reagents and measurements
Infrared spectra were measured on a Nicolet 360 FT-IR instrument using KBr pellet in the 4000~400 cm-1range. Crystalline structure was measured on a BRUKER SMART APEX-CCD diffractometer. Ele- mental analysis was performed by Vario EL Ⅲ elementary analysis instrument. Melting point was measured on a XT4A Melting-Point Apparatus with Micrsocope and uncorrected. Other chemicals were purchased from commercial sources and used without further purification.
2. 2 Synthesis of the title complex
An ethanolic solution (20 mL) of 9-hydroxy- fluorene-9-carboxylic acid (0.5 mmol) was mixed with copper chloride dihydrate (0.5 mmol) at room temperature with continuous stirring. After 1 h, pyridine (1 mL) was added to the mixture slowly, then the solution was filtered. An ethanolic solution of the mixture was stood at room temperature. Upon slowly evaporating ethanol from the solution, blue crystals suitable for X-ray diffraction analysis were isolated one week later. m.p. 131~133 ℃. Anal. Calcd. (%). for Cu(C14H9O3)2(C5H5N)2(C2H5OH)2: C, 65.97; H, 5.24; N, 3.66. Found (%): C, 66.13; H, 5.31; N, 3.57. IR(KBr): 3412(-OH), 3063, 3021(Ar-H), 2965(-CH3), 2872(-CH2), 1631(C=O) cm-1.
2. 3 Crystal structure determination
A blue single crystal of the title compound with dimensions of 0.23mm × 0.20mm× 0.20mm was chosen for X-ray diffraction analysis performed on a BRUKER SMART APEX-CCD diffractometer equipped with a graphite-monochromatic Moradiation (= 0.71073 ?) at 296(2) K. A total of 6766 reflections were collected in the range of 1.78<<25.50o by using ascan mode with 3414 independent ones (int= 0.0232), in which 3155 with> 2() were observed and used in the succeeding refinements. The data set was corrected by SADABS program; the structure was solved by direct methods and expanded by difference Fourier techniques with SHELXS-97[6]. The non-hydrogen atoms were refined anisotropically, and the hydro- gen atoms were added according to the theoretical models. The structure was refined by full-matrix least-squares method on2with SHELXL-97[7].The final refinement gave= 0.0459,= 0.1274 (= 1/[2(F2) +(0.0799)2+ 0.2724],where= (F2+ 2F2)/3),= 1.059, (△/)max= 0.000, (△)max= 0.507 and (△)min= –0.305 e/?3.
3 RESULTS AND DISCUSSION
In order to confirm the configuration of the product, a single crystal of the title compound was cultured for X-ray diffraction analysis. The selected bond lengths and bond angles are shown in Tables 1 and 2, respectively. Table 3 shows the hydrogen- bonding interaction distance. The crystal structure of the title compound is revealed in Fig. 1. Fig. 1 shows that the copper atom is six-coordinated by two oxygen atoms from two different 9-hydroxy-fluo- rene-9-carboxylate ligands, two pyridine nitrogen atoms and two ethanol oxygen atoms, forming a distorted octahedral coordination geometry. In the crystal structure,the bond length of C(6)–C(7) is 1.461(5) ?, ingood agreement with the corres- ponding valueobtained in case of the related fluo- renederivatives. The extensive O–H···Ohydrogen bonding connects the molecules to form a one- dimensional chain structure (Fig. 2). Between adja- cent one-dimensional chains, a two-dimensional layered structure was formed by fluorene ringpacking interaction (Fig. 3). Between the layers, a three-dimensional structure was further generated throughpacking interaction of the pyridine ring (Fig. 4).
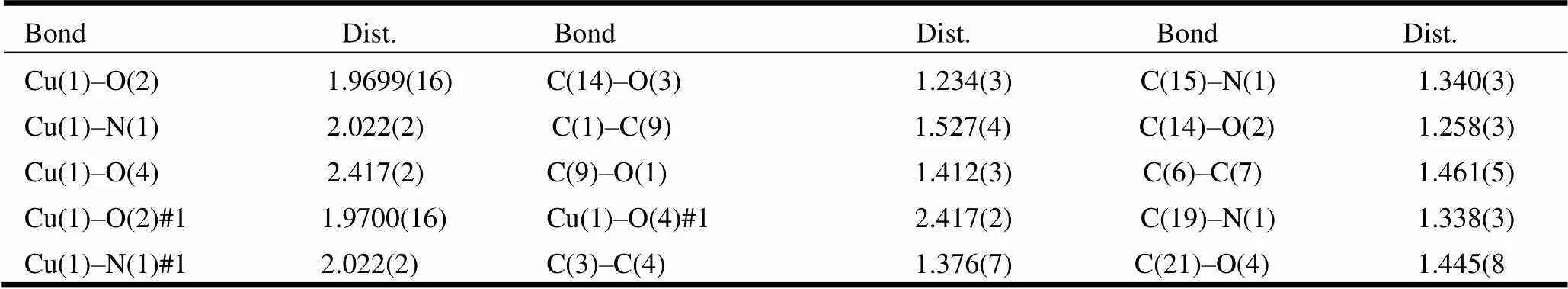
Table 1. Selected Bond lengths (?)
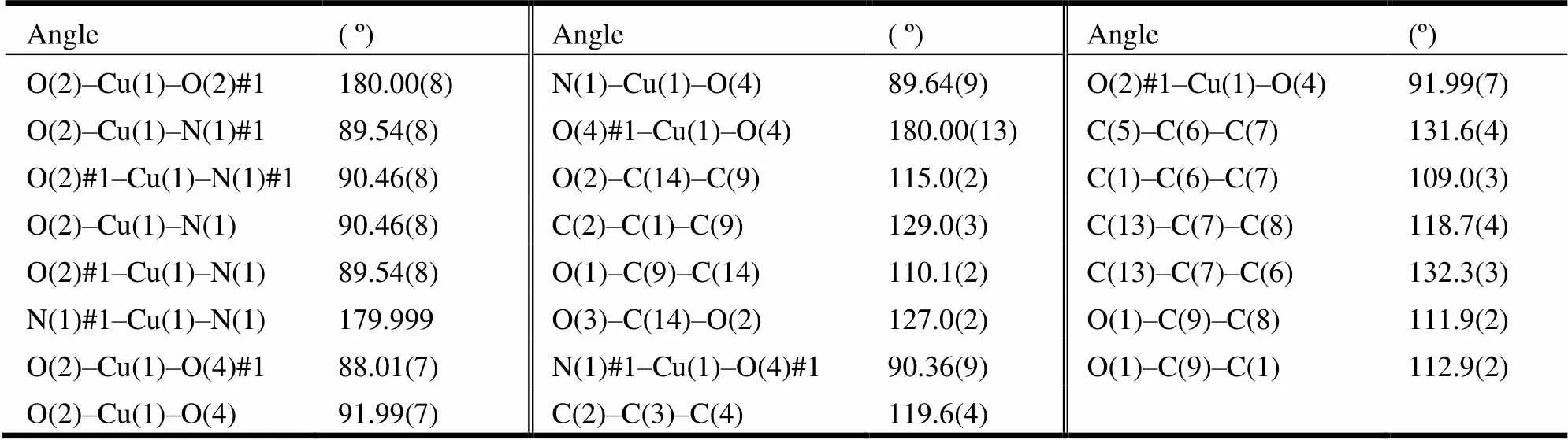
Table 2. Selected Bond Angles (°)
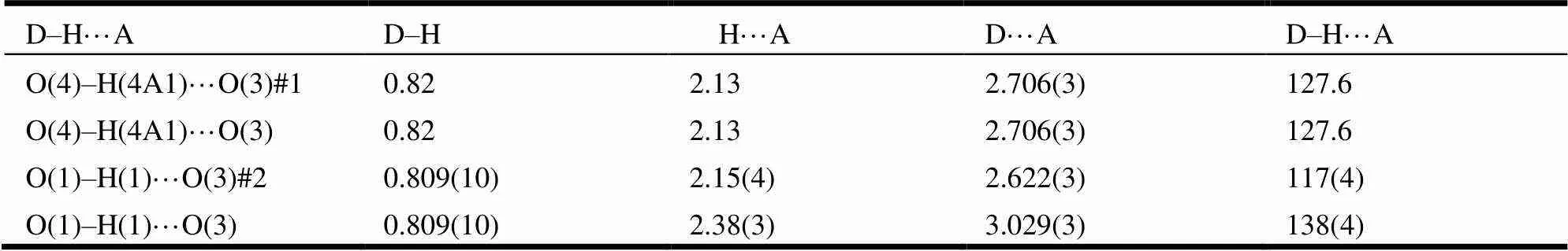
Table 3. Hydrogen-bond Geometry (?, °)
Symmetry codes: #1: –+1, –+2, –+2; #2: –+2, –+1, –+2
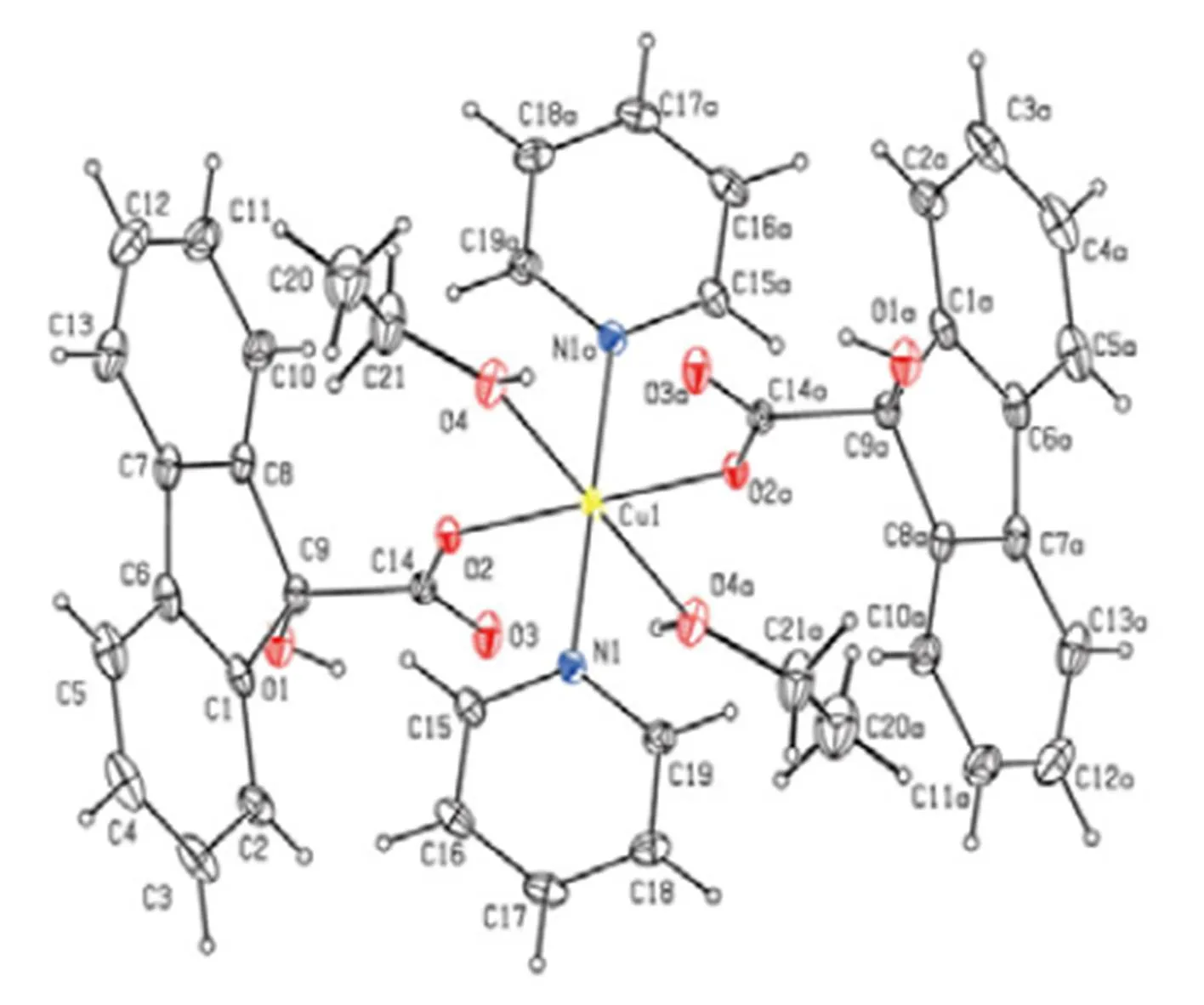
Fig. 1. Molecular structure of the title complex
Fig. 2. One-dimensional chain structure of the title complex
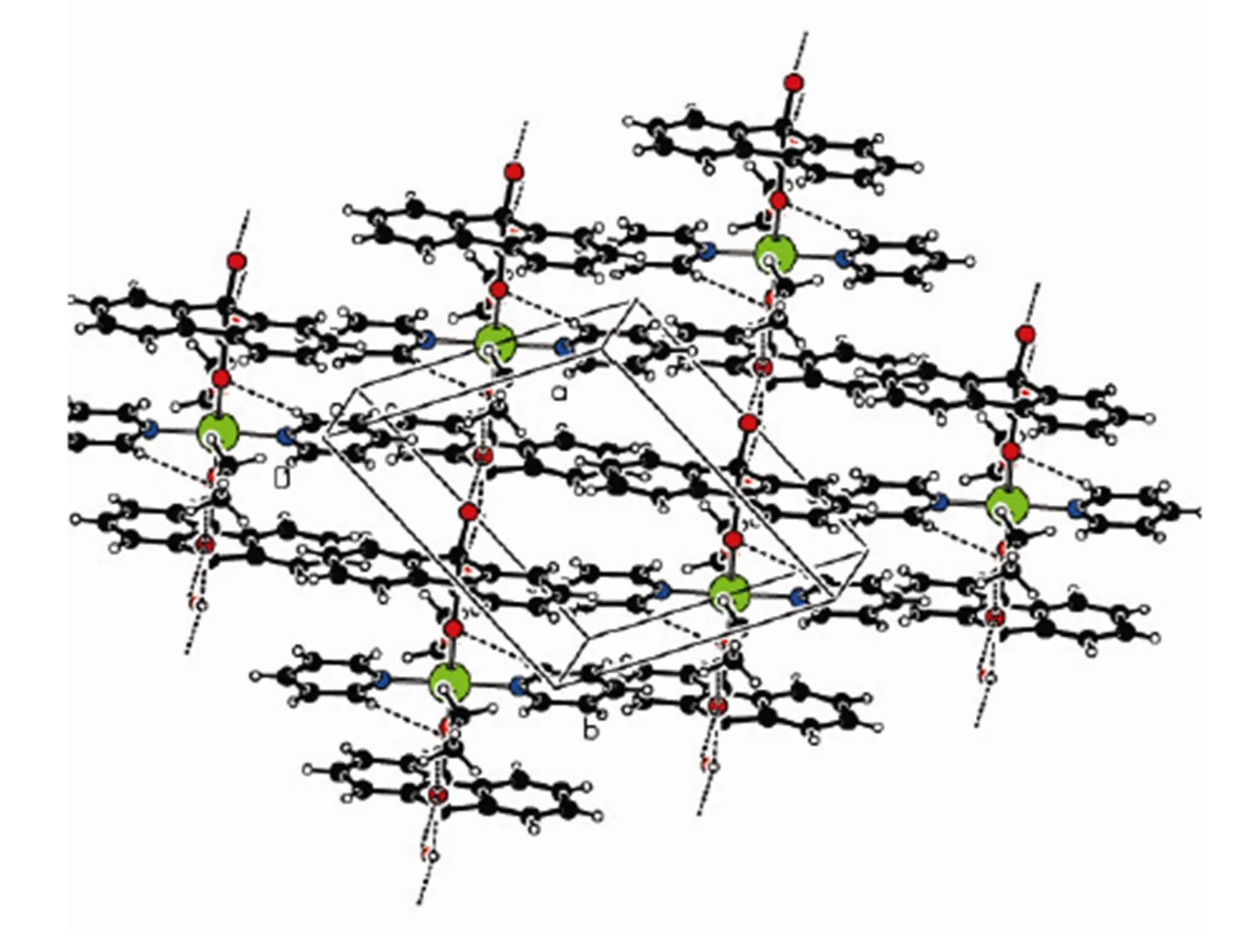
Fig. 3. Two-dimensional layered structure of the title complex
Fig. 4. Three-dimensional structure of the title complex
3. 1 Thermal analyses
Thermal stability of the title complexwas exa- mined by TG analysis under N2atmosphere in the temperature range of 30~500 ℃ (Fig. 5). TG curve of the title complexillustrates that the first weight loss occurring between 135 and 220 ℃ is 13.07% due to the removal of two ethanol molecules (92/764 = 12.04%). At the same time, in the range of 220~315 ℃, there is a 54.68% weight loss owing to the release of two 9-hydroxy-fluorene-9-carboxylic acid molecules (450/764 = 58.90%) and a 54.68% weight loss in the 315~375 ℃ range because of the decomposition of pyridine ligands.
3. 2 Photoluminescent property
Owning to their potential applications in chemical photochemistry, sensors, and electroluminescent (EL) display, luminescent metal-organic frameworks are receiving much attention[8]. In this work, the luminescence properties of compound 1 and the ligand of 9-hydroxy-fluorene-9-carboxylic acid (HHF)were investigated under ambient temperature (Fig. 6). For 1, the emission bands at 460 nm (ex= 316 nm) are observed. The ligand of 9-hydroxy- fluorene-9-carboxylic acid (HHF) exhibits emission band at 491 nm (ex= 332 nm). According to the reported literatures, the emission band of 1 may be interpreted as a ligand-to-metal charge transition (LMCT)[9].
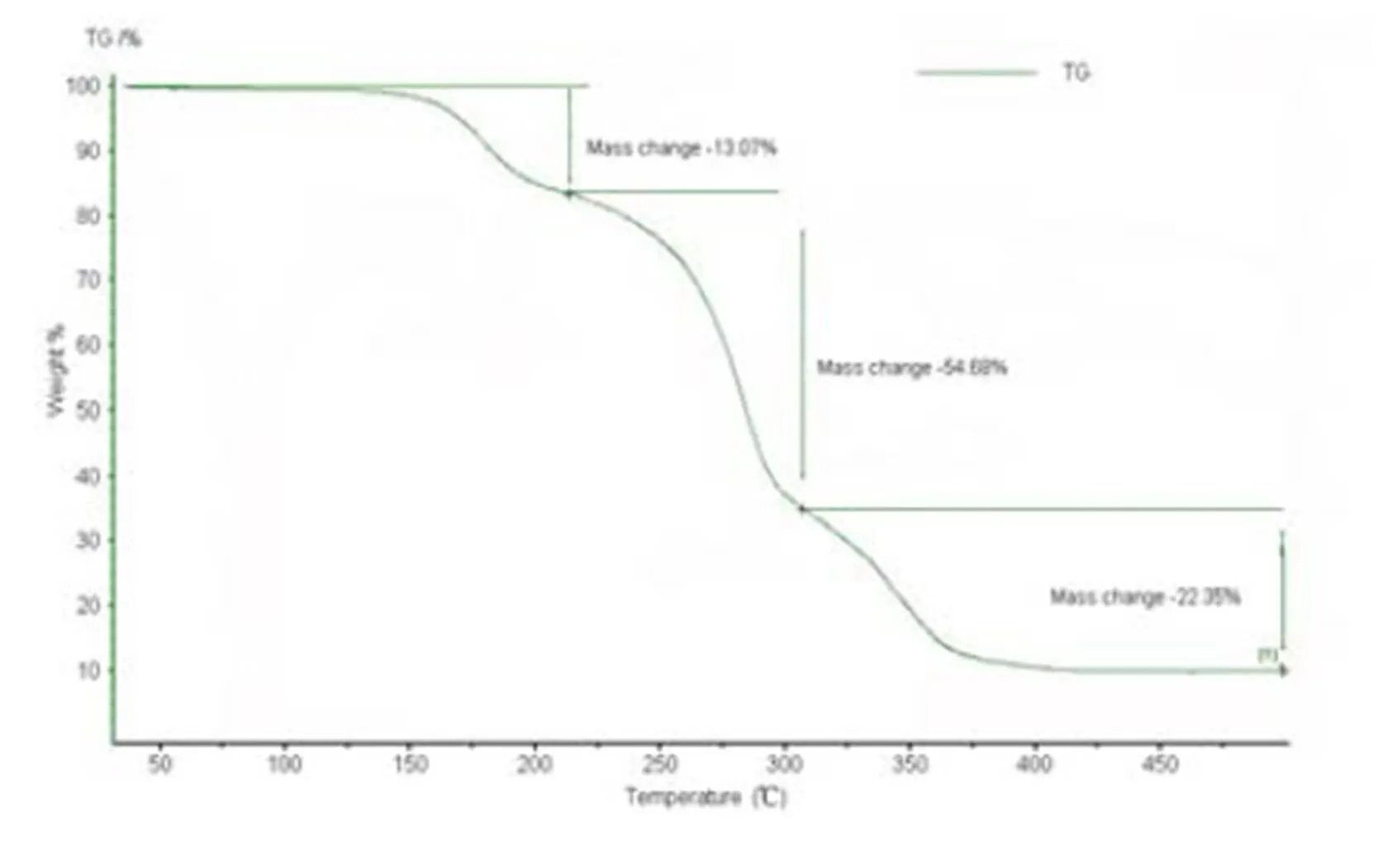
Fig. 5. TG curve of title complex
Fig. 6. Luminescent spectra at room temperature
(1) Feng, F. Synthesis and crystal structure of 9-[3-oxo-1-(4-bromopheny)-3phenypropyl]fluorene.2010, 29, 1760–1763.
(2) Feng, F.; Cui, Z. C.; Duan, Z. C.; Hu, W. B.Synthesis and crystal structure of 2-nitro-9,9-bis(methylpropionate)fluorene.
2012, 31, 1535–1538.
(3) Chen, R. F.; Zhu, R.; Zheng, C.; Liu, S. J.; Fan, Q. L.; Huang, W. Germafluorene conjugated copolymer synthesis and applications in blue-light emittingdiodes and host materials. 2009, 52, 212–218.
(4) Feng, F.; Li, Y. P.; Zhou, H. Y.; Tian, D. T.; Hu, W. B.Synthesis and crystal structure of 10-(3,4-dichlorophenymethylidyne)-9,10- dihydrofluorene.2011, 30, 1111–1114.
(5) Wen, H.; Nai, D.; Zhao, W. D.; Zhao, F. Y. The synthesis of 9-hydroxyfluorene-9-carboxylic acid and its esters.1996, 2, 43-45.
(6) Sheldrick, G. M.,. University of Gottingen, Germany 1990.
(7) Sheldrick, G. M.,. University of Gottingen, Germany 1997.
(8) Liu, Y. C.; Lin, P.; Du, S. W. Two novel homochiral enantiomorphic 3D metal-organic frameworks: synthesis, crystal structure, luminescent and SHG properties.2013, 32, 1509–1516.
(9) Lu, W. G.; Jiang, L.; Feng, X. L.; Lu, T. B. Three 3D coordination polymers constructed by Cd(II) and Zn(II) with imidazole-4,5-dicarboxylate and 4,4?-bipyridyl building blocks.2006, 6, 654–571.
8 October 2013;
2 November 2013 (CCDC 921611)
the Natural Science Foundation of Hubei Province (2012FFB01103),the Hubei Provincial Department of Education (No. Q20131905), the Fund of Ethnic Affairs Commission of China (No. 12HBZ010), the Open Foundation of Key Laboratory of Biologic Resources Protection and Utilization of Hubei Province, Forestry Key Disciplines (PKLHB1303) and the Team Research for Excellent Mid-aged and Young Teachers of Higher Education of Hubei Province (T201006)
.Tel: 15027224903, E-mail: fengfu04971118@163.com
- 結(jié)構(gòu)化學(xué)的其它文章
- Extraction and Crystal Structure of β-Sitosterol
- Quantum Chemical Study on the Structural Characteristics and Stability of AlSn±Clusters①
- Syntheses, Crystal Structures and Catalytic Properties of Copper(II) and Cobalt(II) Complexes Containing 5,6-Dimethylbenzimidazole①
- Syntheses, Structures and Properties of Two 2D Coordination Polymers from 5-(Pyridin-2-yl-methyl)aminoisophthalate①
- Comparative Studies on Two 1,8-Naphthalimide Derivatives with Experimental and Theoretical Methods①
- Synthesis, Crystal Structure and Properties of a New Coordination Polymer Constructed with 3-(4-hydroxypyridinium-1-yl)phthalic Acid

