Syntheses, Crystal Structures and Catalytic Properties of Copper(II) and Cobalt(II) Complexes Containing 5,6-Dimethylbenzimidazole①
ZHANG Zhen-Ming YIN Fu-Jun WANG Li-Ping ZHAO Hong
?
Syntheses, Crystal Structures and Catalytic Properties of Copper(II) and Cobalt(II) Complexes Containing 5,6-Dimethylbenzimidazole①
ZHANG Zhen-Minga, b②YIN Fu-JunbWANG Li-PingaZHAO Hongb
a(221116)b(222005)
Two new complexes, [Cu(DMB)2(NAA)2] (1) and [Co(DMB)2(NAA)2] (2) (DMB = 5,6-dimethylbenzimidazole, HNNA = 1-naphthylacetic acid), were hydrothermally synthesized and characterized by elemental analysis, IR spectroscopy, TGA, PXRD and single-crystal X-ray diffraction analysis. The coordination geometry of the metal centers is a perfect square plane for 1 and a distorted tetrahedron for 2. In compound 1, the mononuclear units are linked by N–H···O hydrogen bonds to form a 1D chain structure, while mononuclear motifs of 2 are extendedN–H···O hydrogen bonding and-stacking interactions into a 2D supramolecular layer. The remarkable catalytic properties of the two complexes for the degradation of Congo red dye have been found.
catalysis, crystal structure, 5,6-dimethylbenzimidazole, supramolecular interaction
1 INTRODUCTION
Over the last ten years, metal-organic coordina- tion complexes have become one of the most studied areas of chemistry and materials science, underpin- ned by a fascination with their potential applications, notably in luminescence, electrical conductivity, magnetism, host-guest chemistry, ion-exchange and catalysis[1–4]. The rational and controllable synthesis of their coordination networks and supramolecular architectures is still a difficult challenge in most cases. Several factors, such as coordination bonds and non-covalent interactions like hydrogen bonds,???and C–H???interactions, may affect the assembly of the resulting compounds. Thus, a key step for the assembly of such complexes is to select suitable organic ligands which possess strong coor- dination ability and can provide the hydrogen bond acceptors/donors and-conjugated system for exten- ding their networks[5–6].
The azo dyes such as Congo red and methyl orange have been the most widely used dyes in the textile industry recently, which are toxic and hazardous to many organisms. The heterogeneous Fenton and Fenton-like process involving the oxida- tion of contaminants like azo dyes by HO· radicals has gained considerable importance in recent times, largely dependent on the transition metal-based catalysts.H2O2works as the precursor of hydroxyl radicals, which mineralizes the dye molecules on the basis of the generation of hydroxyl radicals (·OH) in aqueous[4]. In particular, the development of metal- organic complexes into catalysts could yield several significant advantages, such as enhancing the ca- talyst stability, high catalytic activity, better selec- tivity and easy separability[7-10]. To date, numerous complexes based on benzimidazole derivatives have received increasing attention. The most prominent benzimidazole compound in nature is 5,6-dimethyl- benzimidazole, which is useful to construct metal- organic frameworks (MOFs) and supramolecular coordination complexes in view of its following characteristics: (i) it serves as an axial ligand for cobalt in vitamin B12; (ii) benzimidazole ligands contain both the imidazole ring and a larger conjuga- ted-system, capable of acting as hydrogen bond donors and forstacking interaction to stabilize the supramolecular assembly; (iii) the benzimidazole nitrogen atoms have strong coordination ability[7-10]. Recently, Qin and co-workers have presented a unique supramolecular 4-connected msw/P42/nnm topology, namely [Co(L)0.5(hip)](L = 1,4-bis(5,6- dimethylbenzimidazole)butane, H2hip = 5-hydroxyi- sophthalic acid), which displays remarkable catalytic performances for the degradation of methyl orange on Fenton-like process[9]; Xiao. reported two metal-organic coordination polymers of Co(II) with the molecular formulae [Co(L1)(tp)(H2O)2](1) and[Co(L2)(tp)·H2O](2) (L1= 1,4-bis(benzimidazole- 1-ylmethyl)-benzene, L2= 1,1-(1,4-butane diyl)bis-(5,6-dimethylbenzimidazole), tp = terephthalate). Both compounds display high catalytic activity on the Fenton-like process to the degradation of methyl orange[11]. However, 5,6-dimethylbenzimidazole complexes are less studied and the transition metal complexes bearing both HNAA and DMB ligands are rare[12-15]. In this paper, we present the syntheses, crystal structures and catalytic properties for the degradation of Congo red dye of complexes 1 and 2.
2 EXPERIMENTAL
2. 1 Materials and physical measurements
All the reagents and solvents for syntheses and analyses were commercially available and used as received without further purification. Elemental analyses were performed with a FLASH EA1112 elemental analyzer. The IR spectra as a KBr disk were recorded on a Nicolet NEXUS 470 FTIR spectrometer. Thermal analysis was performed on a NETZSCH STA 409PC thermal analyzer from room temperature to 800 ℃ under nitrogen atmosphere at a heating rate of 10 ℃/min. Powder X-ray diffrac- tion measurement was performed on a Rigaku D/Max-2500 diffractometer operated at 40 kV and 100 mA for a Cu-target tube and a graphite monochromator.
2. 2 Syntheses of complexes 1 and 2
[Cu(DMB)2(NAA)2] (1) A mixture of CuCl2·2H2O (0.17 g, 1.0 mmol), DMB (0.12 g, 1.0 mmol), HNNA (0.19 g, 1.0 mmol), NaOH (0.08 g, 1.0 mmol) and H2O (18 mL) was placed in a Teflon-lined stainless vessel and heated to 160 ℃ for 72 h under autogenous pressure, and then cooled to room temperature at a rate of 5 ℃/h. Blue crystals of 1 were obtained in 38% yield based on Cu. Calcd. (%) for C42H38CuN4O4(= 726.33): C, 69.45; H, 5.27; N, 7.71. Found (%): C, 69.22; H, 5.05; N, 7.49. FTIR (KBr pellet, cm–1): 3430 (w), 3146 (m), 2942 (m), 1595 (s), 1510(m), 1387 (s), 1290 (m), 857 (m), 772 (s), 626 (w), 428 (w).
[Co(DMB)2(NAA)2] (2) Complex 2 was pre- pared using a similar method as for 1 but using CoCl2·6H2O rather than CuCl2·2H2O. Purple crystals of 2 were obtained in 42% yield based on Co. Calcd. (%) for C42H38CoN4O4(= 721.72): C, 69.88; H, 5.31; N, 7.77. Found (%): C, 70.13; H, 5.24; N. 7.59. FTIR (KBr pellet, cm–1): 3430 (w), 3146 (m), 2942 (m), 1590 (s), 1503(m), 1365 (s), 1271 (m), 857 (m), 772 (s), 619 (w), 533(w), 428 (w).
2. 3 Catalysis experiments
The catalytic degradation of dye was carried out in a 250 mL three-necked flask reactor with ma- gnetic stirring. The reaction temperature was con- trolled by circulating constant temperature water. When the temperature was constant, the reaction was initiated by adding 1 mL of 30% (w/w) H2O2to the solution. In a typical run, a given amount of complex 1 or 2 was added into 100 mL Congo red (20 mg/L) solution, keeping the reactions underpH = 6 condition. At a given time interval of 5 min, the solution was taken out and centrifuged to remove the residual catalyst, then analyzed with a UV-Vis 1910 spectrophotometer at an absorption wavelength of 496 nm. Control experiments without the addition of any catalyst or with an equimolar CoCl2·6H2O (or CuCl2·2H2O) with Co(II) (or Cu(II)) involved in the complexes as catalyst were conducted under the same conditions. The degradation efficiency of Congo red is defined as follows[7]:
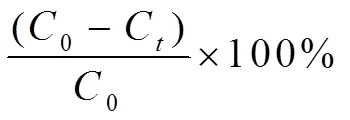
whereC(mg/L) is the initial concentration of Congo red andC(mg/L) is the concentration of Congo red at the reaction time,(min).
2. 4 X-ray data collection and crystal structure determination
The suitable single crystals of the title complexes were mounted on the top of a glass fiber with epoxy cement for X-ray measurement. The crystallographic data collections for complexes 1 and 2 were carried out on a Bruker Smart 1000 CCD diffractometer equipped with a graphite-monochromated Mo-Kα radiation (λ = 0.071073 nm) using an ω-2θ scan mode at 293 K. The absorption corrections were applied using SADABS program[16]. The two structures were solved by direct methods with SHELXS-97 program[17]and refined with SHELXL- 97[18]by full-matrix least-squares fitting on F2. Anisotropic thermal parameters were applied to all non-hydrogen atoms. The organic hydrogen atoms were generated geometrically.A summary of the crystallography data is given in Table 1. The selected bond lengths and bond angles are listed in Tables 2 and 3, respectively.
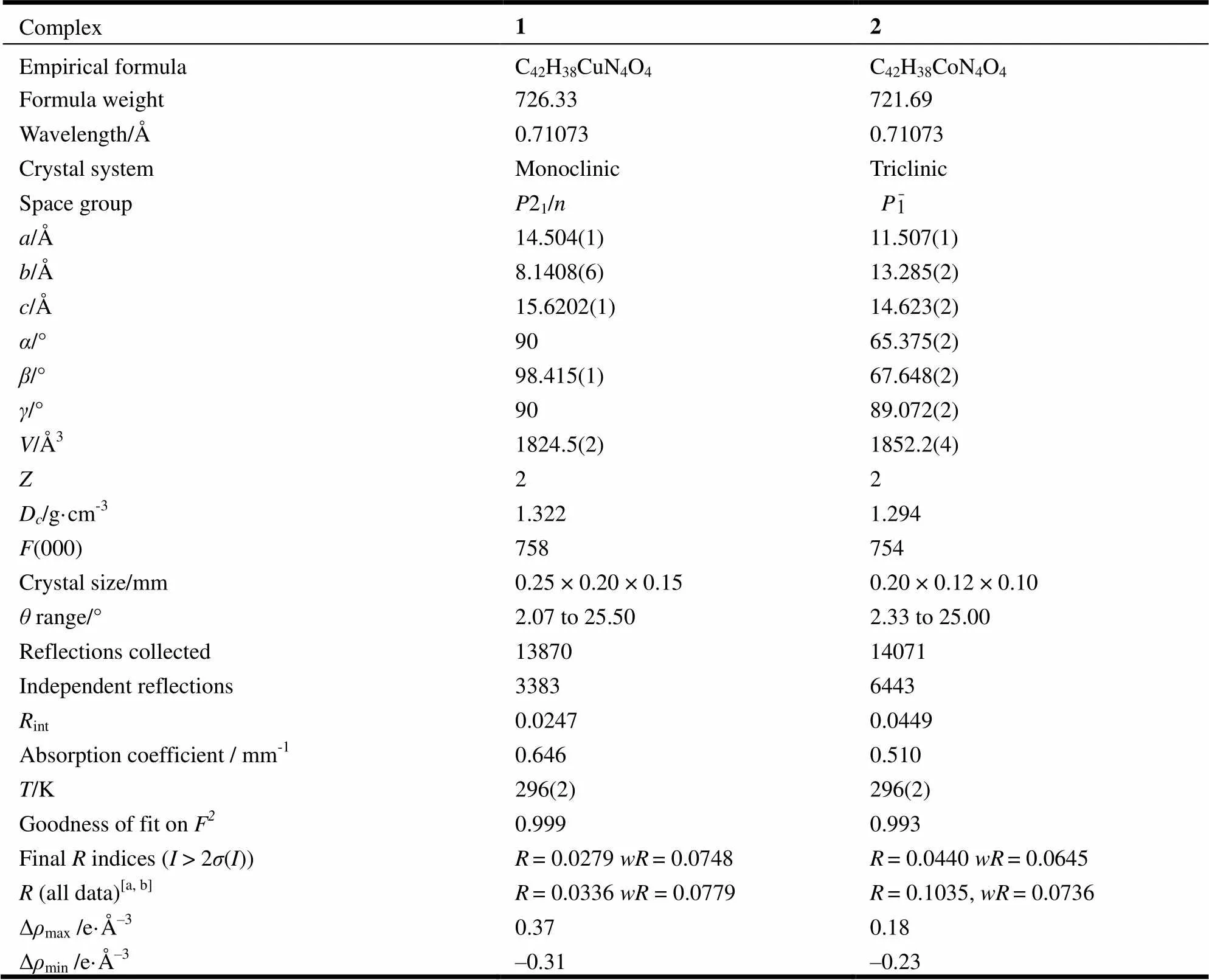
Table 1. Crystallographic Data for Compounds 1 and 2
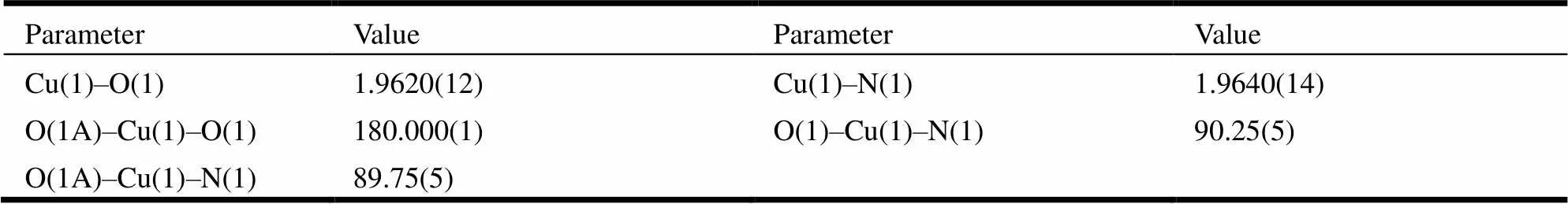
Table 2. Selected Bond Lengths (?) and Bond Angles (°) for 1
Symmetry transformations used to generate the equivalent atoms: A: –+1, –, –+2
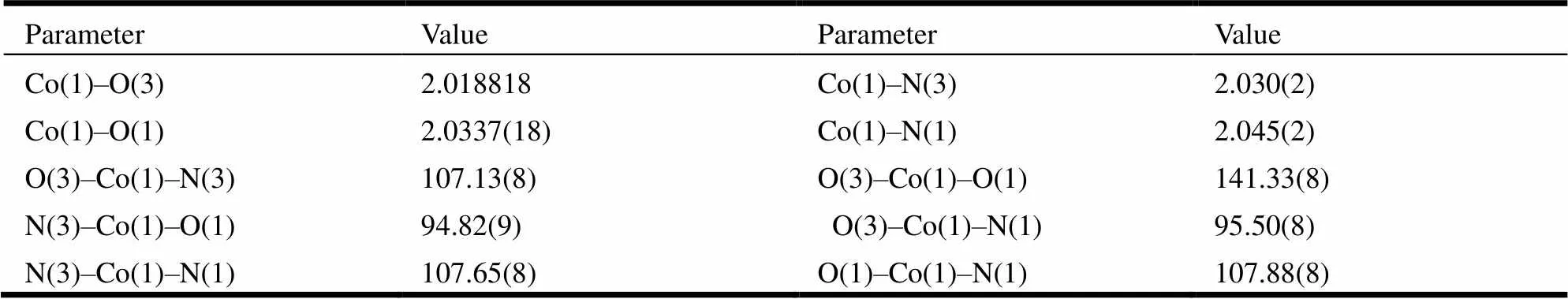
Table 3. Selected Bond Lengths (?) and Bond Angles (°) for 2
3 RESULTS AND DISCUSSION
3. 1 IR spectra of the complexes
In the spectrum of the complexes, the bands at 1510 cm-1for 1 and 1503 cm-1for 2 can be assigned to theC=Nabsorption of benzimidazole rings of the 5,6-dimethylbenzimidazole ligands. The asymmetric and symmetric stretching vibrations of the carboxy- late group from 1-naphthalene acetate were observed at 1595, 1387 cm-1for 1 and 1590, 1365 cm-1for 2, respectively. The separation ofasands(Δare208 and 225 cm-1) imply the presence of monodentate carboxyl groups in both complexes.
3. 2 Crystal structure description of [Cu(DMB)2(NAA)2] (1)
X-ray diffraction analysis revealed that complex 1 crystallizes in the monoclinic21/space group and exhibits a discrete monomolecule. The asymmetric unit of 1 contains half of a Cu(II) cation, one NAA anion, and one DMB ligand. The Cu(II) in 1, as shown in Fig. 1a, is four-coordinated by two N atoms from two terminal DMB ligands (Cu(1)– N(1)/N(1A) = 1.9640(14) ?, symmetry code: A = –+1, –, –+2) and two O atoms from two mono- dentate carboxylate groups of two different NAA ligands (Cu(1)–O(1)/O(1A) = 1.9620(13) ?), showing a perfect square planar geometry with the4value being zero[19]. The twoangles (O(1)– Cu(1)–O(1A), N(1)–Cu(1)–N(1A)) are 180.000(1)° and the fourangles (O–Cu–N) are 89.75(5) and 90.25(5)°. The Cu–N and Cu–O bond lengths have expected values[12]. In 1, two terminal DMB and two terminal NAA are bound to the Cu(II) atom to generate a mononuclear molecule. Adjacent mononuclear molecules connect with each other to form a 1D supramolecular structureN–H···O hydrogen-bonding interactions between benzimi- dazole N(2) atom and the carboxylate O atom (N(2)···O(2B) = 2.758(2) ? , N(2)–H(101)···O(2B) = 171.6(2)°, symmetry code: B = 1–, 1–, 2–), as depicted in Fig. 1b. The Cu···Cu distance of adjacent discrete molecules in the 1D supramolecular struc- ture is 8.140(1) ?. Additionally, theof the ace- tate side chain (C(12)–C(11)–C(5)–C(6)) of each NAA anion is twisted to the naphthalene ring with a torsion angle of 113.61(14)°.
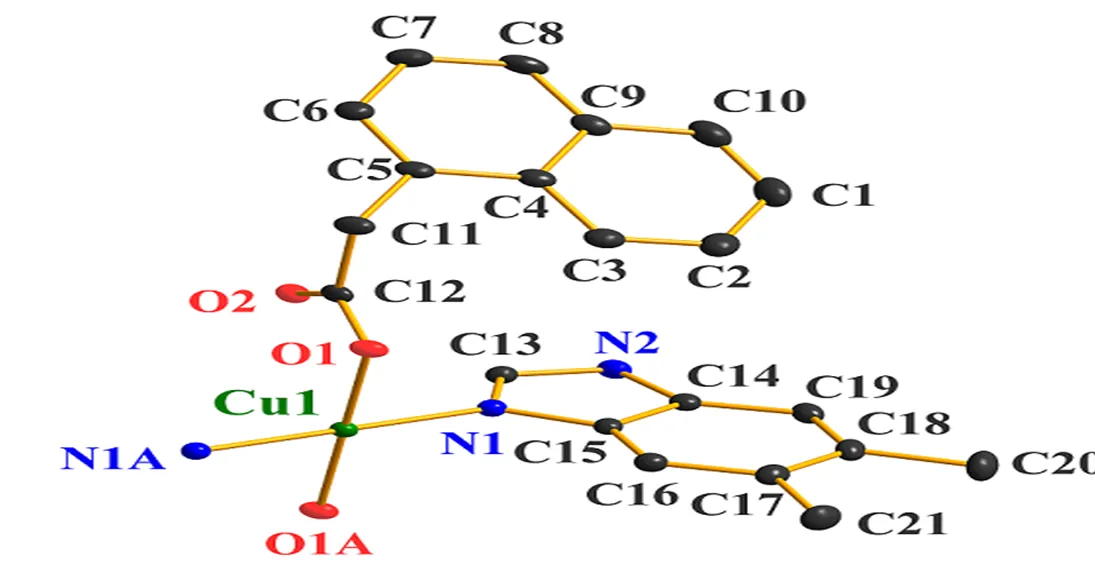
Fig. 1a. Coordination environment of Cu(II) in 1. The hydrogen atoms were omitted for clarity. Symmetry code: A –+1, –, –+2
Fig. 1b. 1D supramolecular chain constructed form the mononuclear molecules through N–H···O hydrogen bonds in 1
3. 3 Crystal structure of [Co(DMB)2(NAA)2] (2)
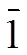
Fig. 2a. Coordination environment of Co(II) in 2. The hydrogen atoms were omitted for clarity
Fig. 2b. View of the supramolecular layer of 2 along theplane. π-π interactions and hydrogen bonds are drawn as dashed lines
3. 4 Thermal analysisand XRD results
The thermogravimetric analyses of samples of 1 and 2 were carried out from 26 to 800 ℃ under a nitrogen atmosphere at a heating rate of 10 ℃ min?1, as shown in Fig. 3a and 3b, respectively. Before 286 ℃ for 1 (252 ℃ for 2), the thermogram does not display any inflexion point, which indicates that the thermostability of the two complexes is high and they do not contain crystal or coordinated water. The TGA curve for 1 exhibits two continuous weight loss stages. The first one of 59.74% around 287~414 ℃ corresponds to the release of two DMB ligands, and the second one in the range of 415~613 ℃ can be attributed to the further decomposition to form CuO as a final product (obsd. 11.3%, calcd. 10.9%). The TGA curve for 2 shows no weight loss between 26 and 252 ℃, indicating that the supramolecular framework of 2 can remain stable up to 252 ℃. The weight loss occurs continuously in the temperature range of 253~582 ℃, assigned to the removal of coordinated DMB ligands and naphthalene acetate to form CoO (obsd. 9.5%, calcd. 10.3%). As shown in Fig. 4, PXRD experiment was carried out for compounds 1 and 2. All of the peaks displayed in the measured patterns closely match those in the simulated patterns generated from single-crystal diffraction data, thus the structure reported is repre- sentative of the bulk materials.
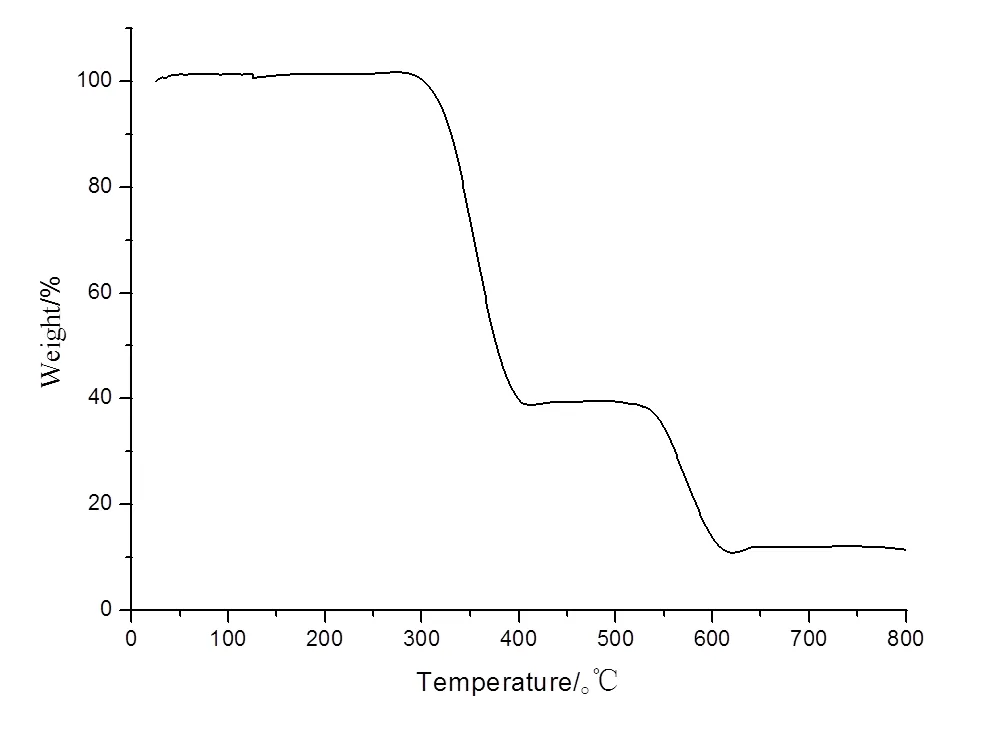
Fig. 3a. TG curve of complex 1
Fig. 3b. Curve of complex 2
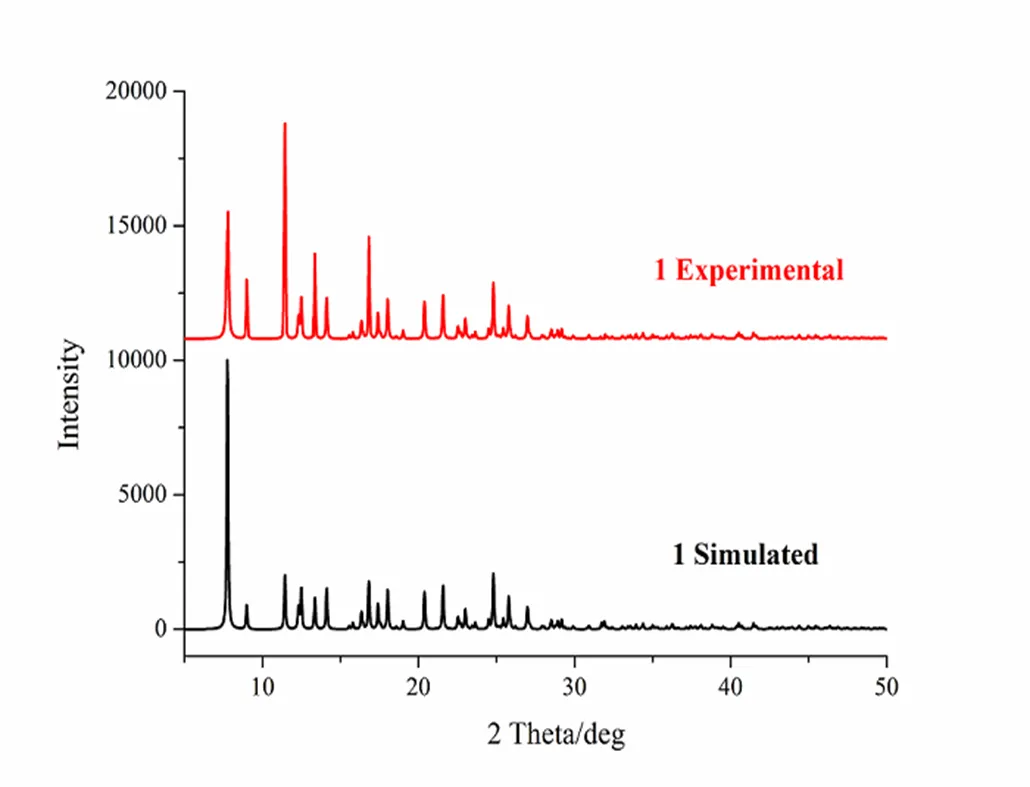
Fig. 4a. XRPD pattern of 1
Fig. 4b. XRPD pattern of 2
3. 5 Catalytic property
The degradation experiments of Congo red by hydrogen peroxide activated with two transition metal complexes were investigated, as shown in Fig. 5. The degradation rates of Congo red were fast in 40 min, in which the degradation efficiency can be achieved at 93.4 and 63.9% using complexes 1 and 2 as the catalysts, respectively; after 40 min, Congo red oxidation proceeded in a gradual manner, in which the degradation efficiencies were up to 96.9 and 66.9% after 80 min, respectively. However, without catalyst, the degradation efficiency of the control experiment was very low with only 12.7% after 80 min. Since M(II) (M = Cu or Co) species may play a key role in these reactions, an equimolarMCl2·6H2O with M(II) contained in the frameworks was investigated. It has been proved that the M2+ions have catalytic effect, but with lower overall degradation efficiency being 76.6% for Cu2+and 55.6% for Co2+in contrast to complexes 1 and 2. Both complexes have high catalytic activities on the degradation of Congo red. The different catalytic performance was not only due to their metal center, but also to the directionality of metal-ligand interac- tion and the angles defined by the mixed ligands and supramolecular interactions, which generate micro cavities/channels that can be accessed from the exterior, allowing mass transfer and the incorpora- tion of substrates inside the crystal[20].
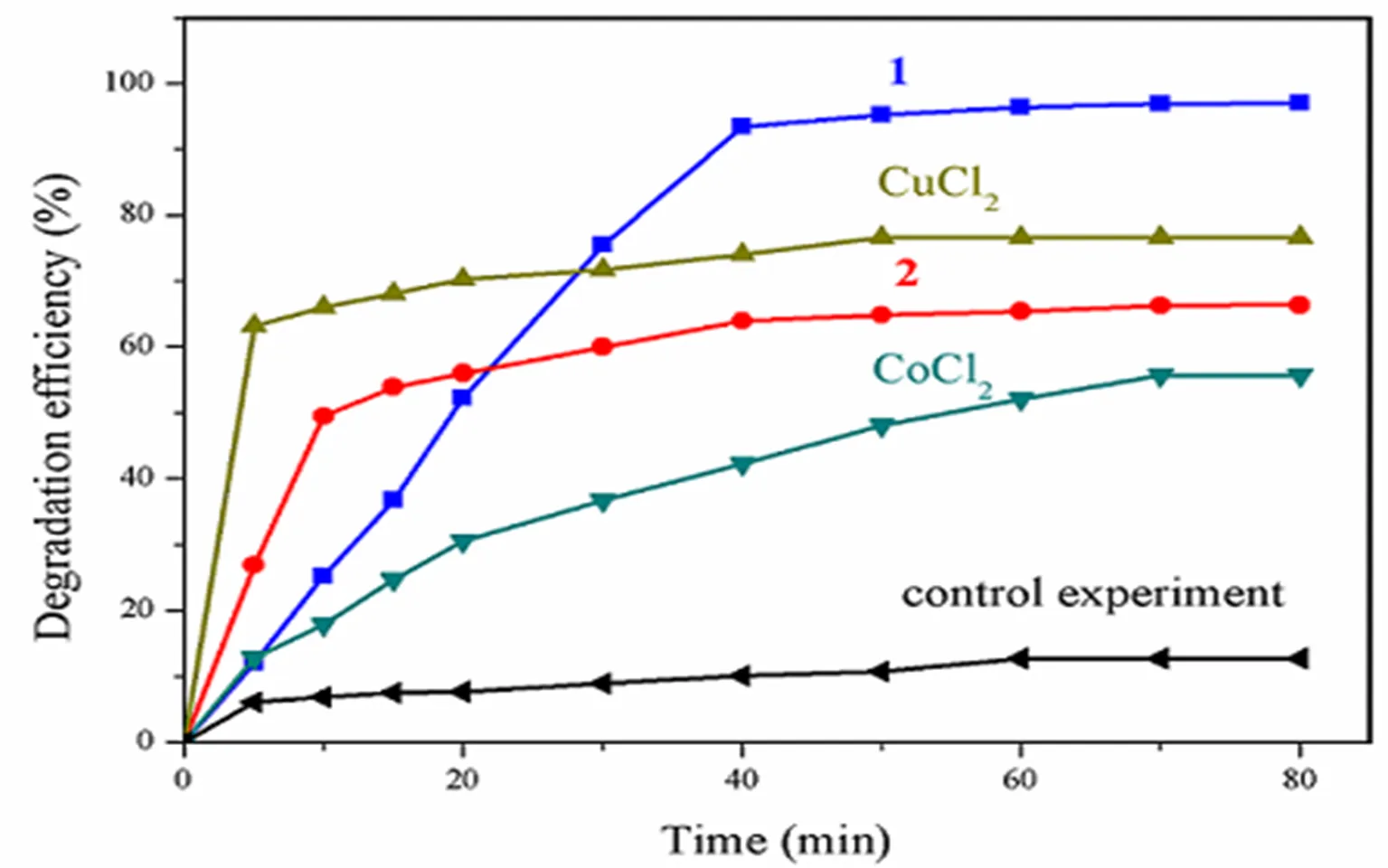
Fig. 5. Experiment results of the catalytic degradation of Congo red
(1) Contreras, R.; Flores-Parra, A.; Mijangos, E.; Téllez, F.; López-Sandoval, H.; Barba-Behrens, N. From mono to polydentate azole and benzazole derivatives, versatile ligands for main group and transition metal atoms...2009253, 1979–1999.
(2) Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T. J.; Li, J. New microporous metal-organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds.2011, 133, 4153–4155.
(3) Tanabe, K. K.; Cohen, S. M. Postsynthetic modification of metal-organic frameworks-a progress report.2011, 40, 498–519.
(4) Cui, G. H.; He, C. H.; Jiao, C. H.; Geng, J. C.; Blatov, V. A. Two metal-organic frameworks with unique high-connected binodal network topologies: synthesis, structures, and catalytic properties.. 2012, 14, 4210–4216.
(5) Cui, G. H.; Liu, T. F.; Peng, X. Structural and spectroscopic study of Cu(II) pyridine-2,6-dicarboxylate complex with 2-ethylimidazole.... 2011, 41, 322–327.
(6) Robin, A. Y.; Fromm, K. M. Coordination polymer networks with O- and N-donors: what they are, why and how they are made?... 2006, 250, 2127–2157.
(7) Geng, J. C.; Qin, L.; Du, X.; Xiao, S. S.; Cui, G. H. Synthesis, crystal structures, and catalytic properties of silver(I) and cobalt(II) coordination polymers based on flexible bis(benzimidazole) with pyridine-2,6-dicarboxylate..... 2012, 638, 1233–1238.
(8) Liu, T. F.; Cui, G. H.; Jiao, C. H.; Li, C. S.; Deng, X. C. Synthesis, characterization and catalysis of a copper(II) MOF based on 1,3,5-benzenetricarboxylic acid and flexible bis(imidazole) ligands.2011, 27, 1417–1422.
(9) Qin, L.; Xiao, S. L.; Zheng, X. H.; Cui, G. H. A new supramolecular net constructed with 2D (4,4) layer subunits displaying unique 4-connected msw/P42/nnm topology: structure, fluorescence and catalytic properties.... 2013, 34, 71–74.
(10) Jiao, C. H.; He, C. H.; Geng, J. C.; Cui, G. H. Synthesis, crystal structures and catalytic properties of two 1D cobalt(II) coordination polymers... 2012, 37, 17–23.
(11) Xiao, S. L.; Qin, L.; Ma, P. J.; Cui, G. H. Cobalt(II)-terephthalate frameworks containing bis(benzimidazole) derivative Co-ligands: syntheses, crystal structures, catalytic and fluorescence properties..... 2013, 639, 1855–1860.
(12) Wang, X. L.; Zhang, J. X.; Hou, L. L.; Zhang, J. W.; Liu, J. C.; Lin, H. Y. Three new complexes based on a flexible bis(benzimidazole) ligand and rigid/flexible organic carboxylateset... 2011, 41, 1579–1585.
(13) Zhang, W. G.; Cui, G. H.; Jiao, C. H.; Geng, J. C. Synthesis, crystal structure and characterization of two cadmium coordination polymers based on a bis(imidazole) ligand.2012, 28, 2494–2500.
(14) Chen, N.; Yang, P.; He, X.; Shao, M.; Li, M. X. Synthesis, structure, luminescence and magnetic property of camphorate coordination polymers containing 5,6-dimethylbenzimidazole auxiliary ligand...2013, 405, 43–50.
(15) Yin, F. J.; Zhao, H.; Xu, X. Y.; Yang, X. J.Synthesis, structure and thermochromic properties of a copper(II) complex based on naphthyl carboxylate and benzimidazolyl ligands.2013, 32, 786–791.
(16) Sheldrick, G. M.. University of G?ttingen, G?ttingen 1996.
(17) Sheldrick, G. M.. University of G?ttingen 1997.
(18) Sheldrick, G. M.. University of G?ttingen1997.
(19) Yang, L.; Douglas, R. P.; Robert, P. H. Structural variation in copper(I) complexes with pyridylmethylamide ligands:structural analysis with a new four-coordinate geometry index,4.. 2007, 955–964.
(20) Corma, A.; Garcia, H.; Xamena, F. X. L. Engineering metal organic frameworks for heterogeneous catalysis... 2010, 110, 4606–4655.
8 August 2013;
17 March 2014 (CCDC 933890 for 1 and 933888 for 2)
Jiangsu province of academic scientific research industrialization projects (JHB2011-60), Public Science and Technology Research Funds Projects of Ocean" (201305007) andLianyungang Science and technology project (CG1110)
. Professor, mainly majoring in functional coordination chemistry. E-mail: zhangzmlyg@126.com
- 結(jié)構(gòu)化學(xué)的其它文章
- Extraction and Crystal Structure of β-Sitosterol
- Quantum Chemical Study on the Structural Characteristics and Stability of AlSn±Clusters①
- Syntheses, Structures and Properties of Two 2D Coordination Polymers from 5-(Pyridin-2-yl-methyl)aminoisophthalate①
- Comparative Studies on Two 1,8-Naphthalimide Derivatives with Experimental and Theoretical Methods①
- Synthesis, Crystal Structure and Properties of a New Coordination Polymer Constructed with 3-(4-hydroxypyridinium-1-yl)phthalic Acid
- Synthesis, Structure and Electrochemical Property of a New 3D Ag(I) Coordination Polymer Constructed by Biphenyl-2,2?,4,4?-tetracarboxylate①

