Synthesis, Structure, and Luminescent Property of a 3-D Strontium Complex [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O①
CHEN Yn-Mei GAO Qin GAO Dn-Dn WANG De-Rong LI Y-Hong② LIU Wei LI Wu
?
Synthesis, Structure, and Luminescent Property of a 3-D Strontium Complex [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O①
CHEN Yan-Meia, bGAO QianaGAO Dan-DanbWANG De-RongbLI Ya-Honga②LIU WeiaLI Wub
a(215123)b(810008)
The title complex [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O (H2pda = pyridine-2,6-dicar- boxylic acid) has been prepared under solvothermal conditions. It has been characterized by X-ray single-crystal diffraction, IR and elemental analysis. The crystal belongs to the monoclinic system, space group21/with= 10.3795(8),= 9.2225(7),= 18.5726(14) ?,= 104.377(2)o,= 1722.2(2) ?3, C28H22N4O20Sr3,M= 997.36, Z = 2,D= 1.923 g/cm3,= 4.722 mm-1,(000) = 984, the final= 0.0269 and= 0.0538. This complex possesses a 3-D structure which is constructed from 1-D chain motifs linked by carboxylate groups. The luminescent property of the title complex has been investigated.
strontium complex, crystal structure, luminescent property, 3-D framework
1 INTRODUCTION
The chemistry of alkaline earth metal complexes is an emerging area of interest, owing to the aesthe- tically pleasing structures that many such complexes possess and potential applications of these com- plexes in the fields of catalysis[1-3], material scien- ce[4-5], and biochemistry[6-7]. The assembly of alka- line earth metal coordination polymers is influenced by many intricate factors[8-17], and one of the most important ones is to select multifunctional organic ligands containing both oxygen and nitrogen atoms[18-30]. Therefore, the-heterocyclic carboxyla- tes are excellent candidates for constructing coor- dination polymers of alkaline earth metals because these metals are highly affinitive for both oxygen and nitrogen donors.
We are interested in the synthesis of coordination polymers of strontium by employing H2pda (H2pda = pyridine-2,6-dicarboxylic acid) as a ligand under solvothermal conditions. The use of H2pda and strontium salts under atmospheric pressure and at room temperature had previously given a mono- nuclear strontium compound[31]and a 1-D linear chain complex[32]. Until now, none of the Sr-com- plexes with 3-D network structures based on pyridine-2,6-dicarboxylic acid have been reported. We envisioned that there might be a multidimen- sional species available under the solvothermal conditions.
With this consideration in mind, we conducted the reaction of H2pda and Sr(OH)2·8H2O under solvo- thermal conditions. A new 3-D strontium complex of the composition [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O has been synthesized. This is the first Sr-complex with 3-D network structure established by SrIIions and pyridine-2,6-dicarboxylic acid. Herein, we re- port the preparation and characterization of the com- plex. The luminescent property of the title complex has been investigated.
2 EXPERIMENTAL
2. 1 Materials and physical measurement
All chemicals were purchased from commercial suppliers and used without further purification. The C, H and N microanalyses were carried out with a Carlo-Erba EA1110 CHNO-S elemental analyzer. FT-IR spectra were recorded from KBr pellets and the range of 400~4000 cm-1on a Nicolet MagNa- IR500 spectrometer. Crystal determination was per- formed with a Bruker SMART APEX ΙΙ CCDC diffractometer equipped with a graphite-monochro- matized Moradiation (= 0.71073 ?). Thermal gravimetric analysis (TGA) was conducted on a NETZSCH STA 449F3 instrument in flowing N2at a heating rate of 5℃/min. Powder X-ray diffrac- tion (PXRD) pattern was recorded on a Rigaku D/Max-2500 diffractometer at 40 kV and 100 Ma with a Cu-target tube and a graphite monochromator. The solid-state luminescence emission/excitation spectra were recorded on a FLS920 fluorescence spectrophotometer equipped with a continuous Xe- 900 xenon lamp and aF900 microsecond flash lamp.
2. 2 Synthesis of [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O
A mixture of H2pda (0.0335 g, 0.2 mmol), Sr(OH)2·8H2O (0.0263 g, 0.1 mmol) and C2H5OH/ H2O = 1:2 (3 mL) was sealed in a Pyrex-tube (8 mL) which was heated at 120 ℃for 24 h. After cooling to room temperature, colorless rod crystals were obtained. Yield: 0.0211 g (70%, based on Sr). Anal. Calcd. (%) for C28H20N4O20Sr3: C, 33.79; H, 2.03; N, 5.63. Found (%): C, 33.82; H, 2.05; N, 5.48. IR (KBr, cm-1): 3477(s), 1611(s), 1569(s), 1467(s), 1435(s), 1380(s), 1273(s), 907(s), 822(s), 767(s), 704(s), 655(s), 535(s).
2. 3 X-ray crystal structure determination
A colourless single crystal of the title complex was carried out with Moradiation using a BRUKER SMART APEX-II CCD diffractometer at 298(2) K using an-2scan mode. In the range of 2.26≤≤28.29o, a total of 11818 reflections were obtained with 4285 unique ones (int= 0.0293) and used in the succeeding refinements. The structure was solved by direct methods and refined by full- matrix least-squares on2using SHELXS-97[33]and SHELXL-97[34]. All non-hydrogen atoms were re- fined anisotropically. The hydrogen atoms were placed in the calculated positions.= 0.0269 and= 0.0538. Goodness-of-fit is 1.003.(Δ)max= 0.398 and (Δ)min=-0.310 e/?3. The selected bond lengths and bond angles of the title complex are listed in Table 1.
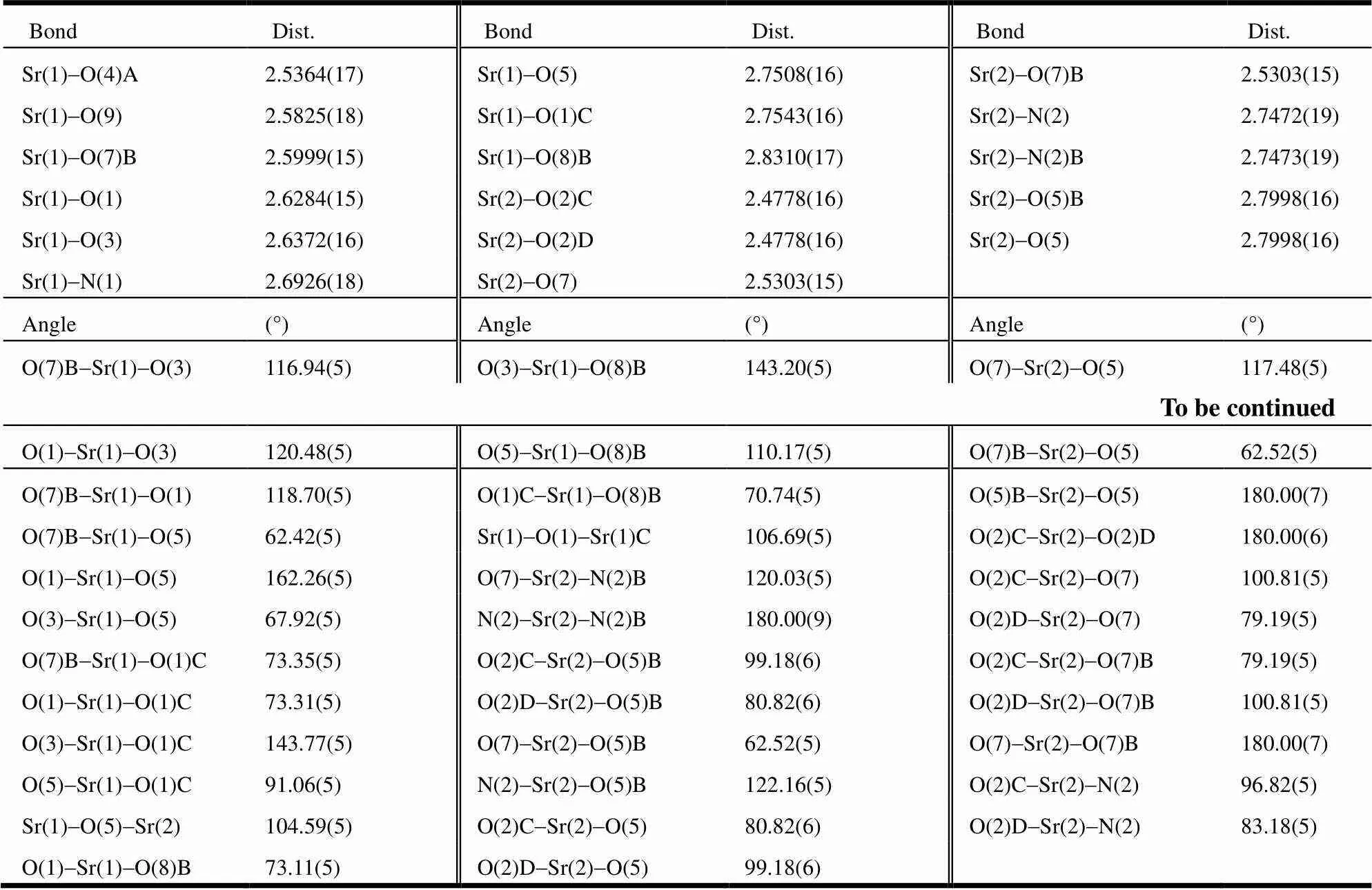
Table 1. Selected Bond Lengths (?) and Bond Angles (°)
Symmetry transformations: A:-+2,-1/2,-+1/2; B:-+1,-,-; C:-+2,-,-; D:-1,,
3 RESULTS AND DISCUSSION
3. 1 Synthesis
The title complex was prepared under solvother- mal conditions. Treatment of a mixture of Sr(OH)2·8H2O and H2pda in C2H5OH/H2O results in the formation of it. It was readily isolated as colorless rod crystals with high yield.
We have explored the importance of solvothermal techniques in this chemistry by attempting the analogous reaction under ‘conventional’ conditions. The synthetic investigation of Sr(OH)2·8H2O/H2pda system in C2H5OH/H2O under ambient reflux conditions gives the known compound [Sr- (C7H3NO4)(H2O)4][32]. The title complex can only be obtained under solvothermal conditions, demon- strating the synthetic novelty of this work.
3. 2 Structure description of [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O
The crystal structure of the title complex is shown in Fig. 1. The crystal structure determination reveals that it crystallizes in the monoclinic crystal system of21/space group. It displays a 3-D network structure. In the asymmetric unit, there are 1.5 characteristic SrIIions. As shown in Fig. 1a, the Sr(1) ion shows a coordination number of ninein a tricapped trigonal prism geometry. It is coordinated by four carboxylic oxygen atoms (O(1), O(3), O(4)A, O(1)C) of three pda2-ligands, three oxygen atoms (O(5), O(7)B, O(8)B) of two Hpda-anions, one nitrogen atom (N1) of a pda2-ligand and one terminal water molecule (O(9)). The distances of Sr(1)-O range from 2.5364(17) to 2.8310(17) ?, in agreement with the reported Sr-O distance[35-39]. The Sr(2) ion shows a coordination number of eight with a slightly distorted dodecahedral geometry. As shown in Fig. 1b, the Sr2 ion is chelated by two Hpda-ligands and is coordinated by two carboxylate oxygen atoms (O(2)C, O(2)D) of two pda2-ligands. The distances of Sr(2)-O range from 2.5303(15) to 2.7998(16) ?, and the Sr(2)-N distance is 2.7472(19) ?. All of them are in accordance with the reported Sr-O and Sr-N distances[35-39]. Two Sr(1) ions are bridged by two2-O(1) atoms. The Sr(2) ion lays on a symmetry center, is connected to two neighboring Sr(1) ions through four2-carboxylic oxygen atoms (2-O(5),2-O(7)B,2-O(5)B,2-O(7)), and is linked to two neighboring Sr(1) ions by two carboxylate groups (O(2)C-C(6)C-O(1)C and O(2)D-C(6)D-O(1)D) to form a 1-D chain structure along the-axis (Fig. 1c). These chains are con- nected by carboxylate groups to form a 3-D network structure (Fig. 1d). An obviousstacking interac- tion between two adjacent pda2-rings of two chains is observed (Fig. 1e), with the center-to-center distance being 3.5499(2) ?. As shown in Fig. 1f, the intermolecular O-H×××O hydrogen contacts between the lattice water molecules (as hydrogen atom donors) and oxygen atoms from coordinated car- boxylate anions of the 3-D network (O(10)-H(10)A···O(8), O(10)-H(10)B···O(4) and O(10)-H(10)B···O(3)) are determined. The O(9) atom of the coordinated water molecule features two kinds of hydrogen bonding interactions; one is intramolecular hydrogen interaction between O(9) and lattice water molecular O(10) and the other is the intramolecular hydrogen interaction between O(9) and carboxylate oxygen atom O(8). More details of hydrogen bonds are summarized in Table 2.
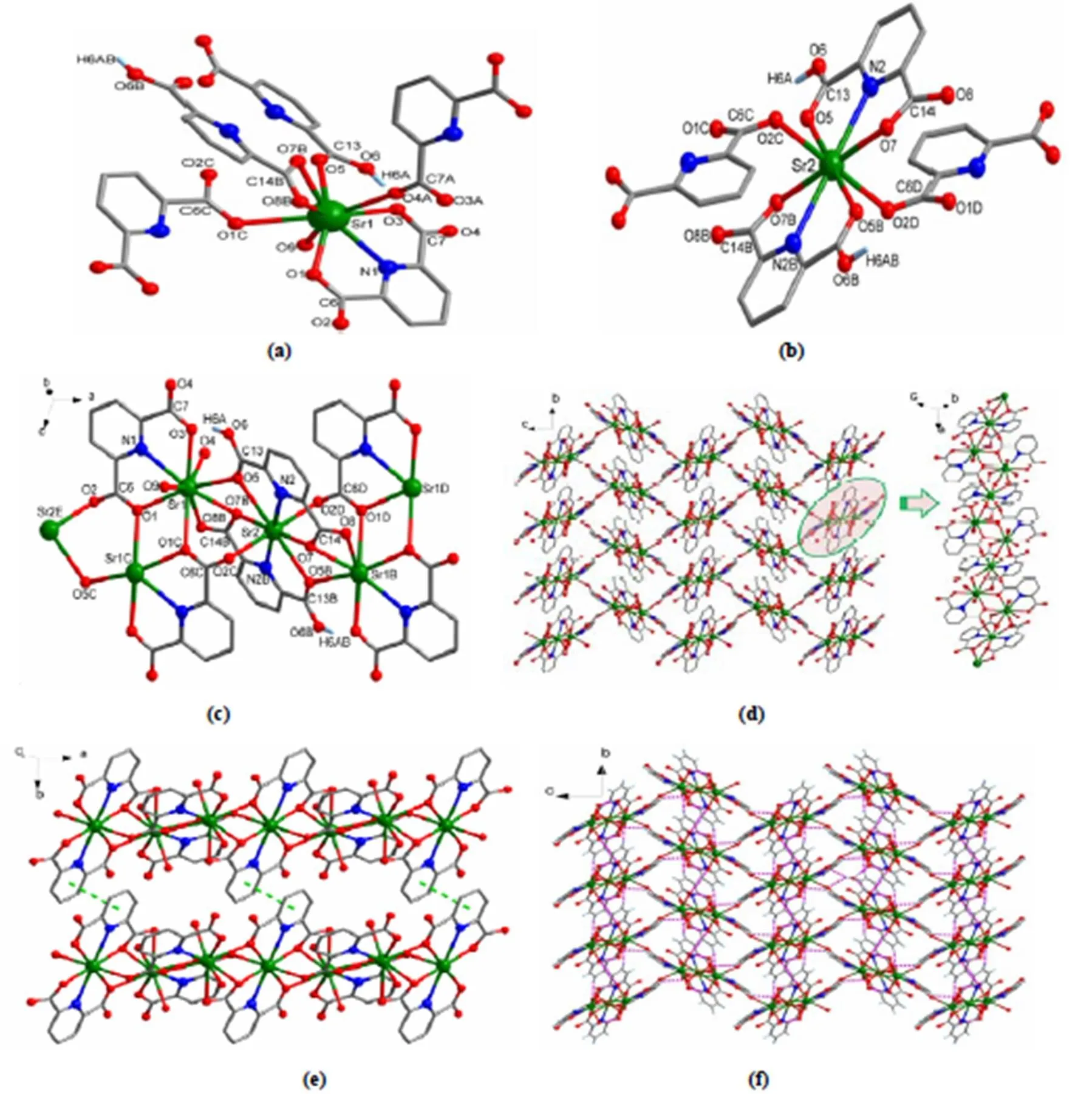
Fig. 1. (a) Coordination environment of the Sr1 ion in the title complex. (b) Coordination environment of Sr2 ion in the title complex. (c) Connection mode of Sr1 and Sr2 ions of the title complex. Symmetry transformations: A:-+2,-1/2,-+1/2; B:-+1,-,-; C:-+2,-,-; D:-1,,; E:+1,,; F:-+2,+1/2,-+1/2. (d) The 3-D framework of the title complex viewed along the-axis. All hydrogen atoms are omitted for clarity. Hydrogen atoms and lattice water molecules are omitted for clarity. (e) Thestacking interactions between the chains. (f) Hydrogen interactions between the 3-D framework and lattice water molecules of 1 along the-axis. Color codes: green, Sr; red, O; blue, N; gray, C; pale blue, H
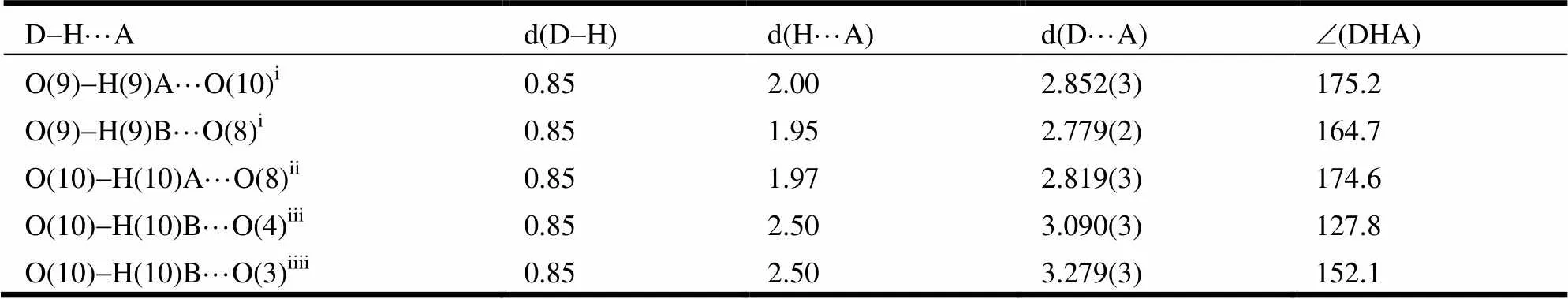
Table 2. Hydrogen Bond Lengths (?) and Bond Angles (°)
Symmetry transformations used to generate the equivalent atoms: i:+1,,; ii:-,-+1,-; iii:-1,,; iiii:-+1,+1/2,-+1/2
The title complex joins a small family of stron- tium complexes with carboxylic acids as li- gands[18-32].To the best of our knowledge, the title complex is thefirst SrII3-D framework supported by H2pda.
3. 3 Powder X-ray diffraction (PXRD) and thermogravimetry
In order to check the phase purity of the complex, the powder X-ray diffraction (PXRD) has been measured and compared with that simulated from the single-crystal structure. As can be seen from Fig. 2, the experimental PXRD patterns and simulated peaks match well, indicating the purities of the sample.
The thermal stability of the title complex was investigated using thermogravimetric analysis. As shown in Fig. 3, the first weight loss of the title complex takes place between 37.2 and 145.1 ℃, with a weight loss of 6.81% (calculated: 7.22%), which corresponds to the removal of all lattice and coordinated water molecules. Above 164.8 ℃, the structure begins to collapse and decompose to SrCO3, with the residual quality to be 46.77% (calcd. 44.2%)
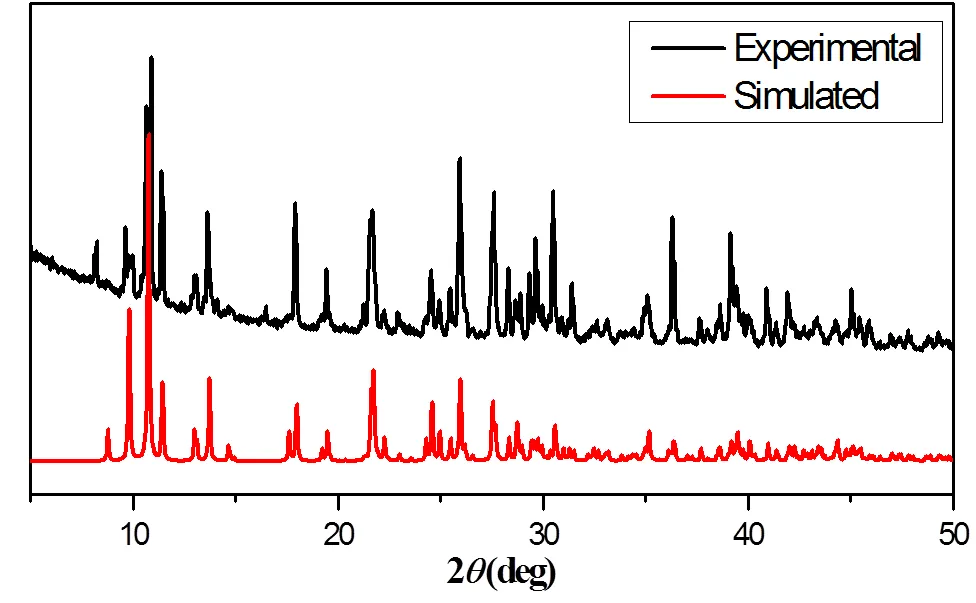
Fig. 2. Powder XRD patterns of the title complex
Fig. 3. TGA diagram for the title complex
3. 4 IR spectra
The IR spectral analysis shows broad band in the range of 3099~3598 cm-1, which can be attributed to the stretching vibrations of(O–H), revealing the presence of water molecules. Theas(OCO) antistre- tching vibrations ands(OCO) stretching vibrations of carboxylate groups of H2pda ligands are located at 1611 and 1467 cm-1, indicating the carboxylate coordination[39]. The strong bands at 1715 and 1569 cm-1can be assigned as(C=O) and(C=N), respec- tively. The absorption bands assigned to the Sr–O and Sr–N stretching frequencies were observed at 535 and 416 cm-1, respectively. These results indi- cate that the ligands coordinate to the SrIIion via oxygen of the carboxyl and nitrogen of pyridine[40-41].
3. 5 Luminescent property
The luminescent properties of [Sr3(pda)2- (Hpda)2(H2O)2]n·2nH2O and the H2pda ligand were investigated in the solid state at room temperature. As shown in Fig. 4,upon excitation at 343 nm, the ligand H2pda exhibits emission maxi- mum at 405 nm, which can probably be attributed to the*-transitions[42-44]. It can be seen that complex [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2Oexhibits radiation emission maxima at 540 nm (ex= 500 nm). The emission bands exhibit red-shift in comparison with the ligand H2pda. Such fluorescent behavior suggests that the emission is mainly attributed to metal perturbed-* intraligand fluorescence[45].
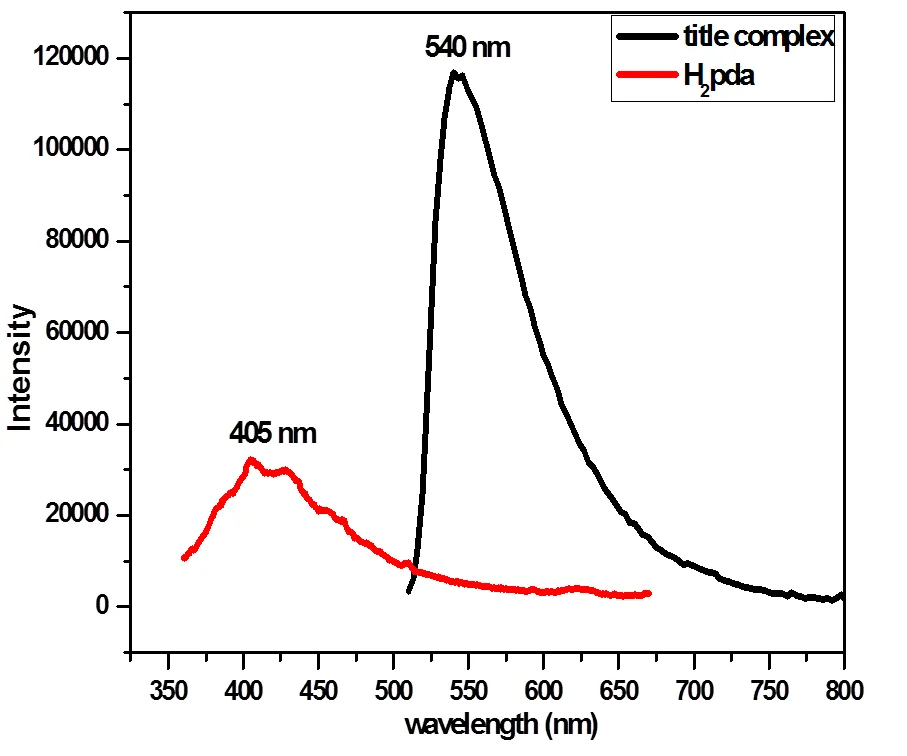
Fig. 4. Room-temperature emission spectra of the title complex (ex= 500 nm) and H2pda (ex= 343 nm) in solid state (emission slit = 1 nm)
4 CONCLUSION
In summary, a new 3-D strontium complex of the composition [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O has been prepared under solvothermol conditions. The thermal and luminescent properties have been inves- tigated, and it shows strong green fluorescence emission. The versatile coordination and connection modes of SrIIions prompt us to continue the research of Sr-containing coordination chemistry. We are pursuing the ways of producing interesting coor- dination polymers of other alkaline earth metals supported by the H2pda ligand. Such work is in progress and will be reported soon.
(1) Harder, S. Intramolecular C–H activation in alkaline-earth metal complexes.2003, 42, 3430–3434.
(2) Westerhausen, M.; Schneiderbauer, S.; Kneifel, A. N.; S?ltl, Y.; Mayer, P.; N?th, H.; Zhong, Z.; Dijkstra, P. J.; Feijen, J. Organocalcium compounds with catalytic activity for the ring-opening polymerization of lactones.. 2003, 3432–3439.
(3) Kobayashi, S.; Yamashita, Y. Alkaline earth metal catalysts for asymmetric reactions.. 2011, 44, 58–71.
(4) Fromm. K. M. Recent advances in tailoring the aggregation of heavier alkaline earth metal halides, alkoxides and aryloxides from non-aqueous solvents.2006, 5103–5112.
(5) Fromm, K. M.; Gueneau, E. D.; Bernerdinelli, G.; Goesmann, H.; Weber, J.; Mayor-López, M. J.; Boulet, P.; Chermette, H. Understanding the formation of new clusters of alkali and alkaline earth metals:? a new synthetic approach, single-crystal structures, and theoretical calculations.. 2003, 125, 3593–3604.
(6) Zhang, X. F.; Deng, Z. P.; Huo, L. H.; Feng, Q. M.; Gao, S. Four alkali-induced 3D strontium(II) coordination polymers constructed from imidazole-4,5-dicarboxylate: syntheses, crystal structures, and properties.2012, 5506–5514.
(7) Fromm, K. M. Coordination polymer networks with-block metal ions.2008, 252, 856–885.
(8) Wang, X. L.; Qin, C.; Wang, E. B.; Li, Y. G.; Su, Z. M.; Xu, L.; Carlucci, L. Entangled coordination networks with inherent features of polycatenation, polythreading, and polyknotting..2005, 44, 5824–5827.
(9) Forster, P. M.; Burbank, A. R.; Livage, C.; Ferey, G.; Cheetham. A. K. The role of temperature in the synthesis of hybrid inorganic-organic materials: the example of cobalt succinates..2004, 368–369.
(10) Tong, M. L.; Kitagawa, S.; Chang, H. C.; Ohba, M. Temperature-controlled hydrothermal synthesis of a 2D ferromagnetic coordination bilayered polymer and a novel 3D network with inorganic Co3(OH)2ferrimagnetic chains.. 2004, 418–419.
(11) Zheng, P. Q.; Ren, Y. P.; Long, L. S.; Huang, R. B.; Zheng, L. S. pH-Dependent assembly of keggin-based supramolecular architecture..2005, 44, 1190–1192.
(12) Go, Y. B.; Wang, X. Q.; Anokhina, E. V.; Jacobson, A. J. Influence of the reaction temperature and pH on the coordination modes of the 1,4-benzenedicarboxylate (BDC) ligand:? a case study of the NiII(BDC)/2,2¢-bipyridine system.. 2005, 44, 8265–8271.
(13) Chen, C. Y.; Cheng, P. Y.; Wu, H. H.; Lee, H. M. Conformational effect of 2,6-bis(imidazol-1-yl)pyridine on the self-assembly of 1D coordination chains:? spontaneous resolution, supramolecular isomerism, and structural transformation.. 2007, 46, 5691–5699.
(14) Wang, R. H.; Yuan, D. Q.; Jiang, F. L.; Han, L.; Gong, Y. Q.; Hong, M. C. Anion effect on the structural conformation of tetranuclear cadmium(II) complexes..2006, 6, 1351–1360.
(15) Zhang, L. Y.; Zhang, J. P.; Lin, Y. Y.; Chen, X. M. Syntheses, structures, and photoluminescence of three coordination polymers of cadmium dicarboxylates..2006, 6, 1684–1689.
(16) Chen, W. X.; Wu, S. T.; Long, L. S.; Huang, R. B.; Zheng, L. S. Construction of a three-fold parallel interpenetration network and bilayer structure based on copper(II) and trimesic acid.. 2007, 7, 1171–1175.
(17) Liu, C. M.; Gao, S.; Zhang, D. Q.; Zhu. D. B. Three-dimensional eight- or four-connected metal-organic frameworks tuned by hydrothermal temperatures.. 2007,7, 1312–1317.
(18) Ptasiewicz-B?k, H.; Leciejewicz, J. The crystal structure of a strontium(II) complex with pyrazine-2,6-dicarboxylate and water Ligands.2003, 56, 223–229.
(19) Dan, M.; Cheetham, A. K.; Rao, C. N. R. Diverse structures and dimensionalities in hybrid frameworks of strontium and lanthanum with isomeric dihydroxybenzoates.. 2006, 45, 8227–8238.
(20) Fei, Z. F.; Geldbach, T. J.; Zhao, D. B.; Scopelliti, R.; Dyson, P. J. A nearly planar water sheet sandwiched between strontium-imidazolium carboxylate coordination polymers.. 2005, 44, 5200–5202.
(21) Casnati, A.; Baldini, L.; Pelizzi, N.; Rissanen, K.; Ugozzoli, F.; Ungaro, R.Strontium complexes of calixarene amides in the solid state: structural dependence on the ligand size and on the counter ions..2000, 3411–3415.
(22) Ptasiewicz-bak, H.; Leciejewicz, J. Strontium ions bridged by pyrazine-2,5-dicarboxylic acid and water molecules make a three-dimensional polymeric structure of triaquomono (-pyrazine-2,5-dicarboxylato) strontium(Ii) sihydrate crystals.1998, 44, 237–246.
(23) Murugaval, R.; Karambelkar, V. V.; Anantharaman, G.; Walawalkar, M. G. Synthesis, spectral characterization, and structural studies of 2-aminobenzoate complexes of divalent alkaline earth metal ions:? X-ray crystal structures of [Ca(2-aba)2(OH2)3]∞, [{Sr(2-aba)2(OH2)2}·H2O]∞, and [Ba(2-aba)2(OH2)]∞(2-abaH = 2-NH2C6H4COOH).. 2000, 39, 1381–1390.
(24) Pan, L.; Frydel, T.; Sander, M. B.; Huang, X.; Li, J. The effect of pH on the dimensionality of coordination polymers.2001, 40, 1271–1283.
(25) Arlin, J. B.; Florence, A. J.; Johnston, A.; Kennedy, A. R.; Miller, G. J.; Patterson, K. Systematic data set for structure-property investigations: solubility and solid-state structure of alkaline earth metal salts of benzoates.. 2011, 11,1318–1327.
(26) Li, S. J.; Song, W. D.; Miao, D. L.; Hu, S. W.; Ji, L. L.; Ma, D. Y. Synthesis, structures, and properties of a series of new coordination polymers built from 2-ethyl-1H-imidazole-4,5-dicarboxylate ligand.2011, 637, 1246–1252.
(27) Murugavel, R.; Korah, R. Structural diversity and supramolecular aggregation in calcium, strontium, and barium salicylates incorporating 1,10-phenanthroline and 4,4‘-bipyridine:? probing the softer side of group 2 metal ions with pyridinic ligands.. 2007, 46,11048–11062.
(28) Wang, W. Y.; Yang, Z. L.; Wang, C. J.; Lu, H. J.; Zang, S. Q.; Li, G. 2-Phenyl-4,5-imidazole dicarboxylate-based metal-organic frameworks assembled under hydro(solvo)thermal conditions.2011, 13, 4895–4902.
(29) Li, Z. F.; Chen, C. J.; Yan, L. H.; Li, G.; Wu, C. J.; Lu, H. J. Three main group metal coordination polymers built by 2-propyl-1H-imidazole-4,5-dicarboxylate.2011, 377, 42–49.
(30) Wang, L. D.; Tao, F.; Cheng, M. L.; Liu, Q.; Han, W.; Wu, Y. J.; Yang, D. D.; Wang, L. J. Syntheses, crystal structures, and luminescence of two main-group metal complexes based on 3,4-pyrazoledicarboxylic acid.2012, 65, 923–933.
(31) Soleimannejad, J.; Aghabozorg, H.; Hooshmand, S.; Adams, H. 4,4′-Bipyridinediium triaquabis(pyridine-2,6-dicarboxylato)strontium(II) trihydrate.2007, 63, m3089–m3090.
(32) Palmer, K. J.; Wong, R. Y.; Lewis, J. C. The crystal structure of strontium dipicolinate tetrahydrate, Sr·C7H3NO4·4H2O.1972, 28, 223–228.
(33) Sheldrick, G.M.University of G?ttingen, Germany1997.
(34) Sheldrick, G.M.. University of G?ttingen, Germany1997.
(35) Garkani-Nejad, Z.; Ahmadvand, M. Simultaneous estimation of stability constants of Mg, Ba, Ca, and Sr complexes using a small subset of molecular descriptors.2011, 64, 2466–2479.
(36) Boyle, T. J.; Tafoya, C. J.; Scott, B. L.; Ziller, J. W. Coordination compounds of strontium: syntheses, characterizations, and crystal structures of [Sr(-ONc)2(HONc)4]2and Sr5(4-O)(3-ONep)4(-ONep)4(HONep)(solv)4(ONc = O2CCH2CMe3; Nep = CH2CMe3; solv = tetrahydrofuran or 1-methyl-imidazole).2000, 51, 361–378.
(37) Wang, X.; San, L. K.; Nguyen, H.; Shafer, N. M.; Hernandez, M. T.; Chen, Z. Alkaline earth metal-organic frameworks supported by ditopic carboxylates.2013, 66, 826–835.
(38) Yang, G. W.; Wang, B. J.; Shen, Z. T.; Li, Q. Y.; Ji, C.; Shen, X. F.; He, M. H. Coordination architectures and luminescent properties of 5-(pyrimidyl)tetrazolate (Hpmtz) with group 2 metal ions.2012, 65, 2657–2670.
(39) Dalai, S.; Rana, A.; Chowdhuri, D. S.; Bera, M.; Zangrando, E. Cubane-like water clusters in a 3D supramolecular network of zinc with pyridine-2,6-dicarboxylic acid and 5-aminotetrazole.2010, 363, 1052–1055.
(40) Luo, Y. M.; Li, J.; Xiao, L. X.; Tang, R. R.; Tang, X. C. Synthesis, characterization and fluorescence properties of Eu(III) and Tb(III) complexes with novel mono-substituted beta-diketone ligands and 1,10-phenanthroline.2009, 72, 703–708.
(41) Chen, M. L.; Hou, Y. H.; Xia, W. S.; Zhou, Z. H. Structures and thermal properties of strontium and barium 1,3-propanediaminetetraacetates.2013, 66, 1906–1915.
(42) Guo, X. F.; Feng, M. L.; Xie, Z. L.; Li, J. R.; Huang, X. Y. The first examples of lanthanide selenite-carboxylate compounds: syntheses, crystal structures and properties.2008, 3101–3106.
(43) Li, S. Z.; Zhang, D. D.; Guo, Y. Y.; Ma, P. T.; Zhao, J. W.; Wang, J. P.; Niu, J. Y. A series of 3D rare-earth-metal-organic frameworks with isolated guest feggin dilicotungstate gragments as snion templates.2011, 5397–5404.
(44) Wu, W. P.; Wang, Y. Y.; Wang, C. J.; Wu, Y. P.; Liu, P.; Shi, Q. Z. Structural characterization and luminescence behavior of a 2D silver (I) coordination polymer assembled from pyridine-2,6-dicarboxylic acid N-oxide.2006, 9, 645–648.
(45) Hu, F. L.; Yin, X. H.; Lu, J.; Mi, Y.; Zhuang, J. C.; Luo, W. Q. Crystal structure, bioactivities, and electrochemistry properties of four diverse complexes with a new pyrazole ligand.2010, 63, 263–272.
8 October 2013;
6 November 2013 (CCDC 932761)
the National Natural Science Foundation of China (21272167 and 21201127), a project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institution, Graduate Education Innovation Project in Jiangsu Province (CXZZ12_0808), Qinghai Science & Technology Department of China (2011-G-208 and 2011-Z-722), and KLSLRC (KLSLRC-KF-13-HX-1)
. Born in 1968, professor, majoring in organometallic chemistry. E-mail: liyahong@suda.edu.cn
- 結(jié)構(gòu)化學(xué)的其它文章
- Extraction and Crystal Structure of β-Sitosterol
- Quantum Chemical Study on the Structural Characteristics and Stability of AlSn±Clusters①
- Syntheses, Crystal Structures and Catalytic Properties of Copper(II) and Cobalt(II) Complexes Containing 5,6-Dimethylbenzimidazole①
- Syntheses, Structures and Properties of Two 2D Coordination Polymers from 5-(Pyridin-2-yl-methyl)aminoisophthalate①
- Comparative Studies on Two 1,8-Naphthalimide Derivatives with Experimental and Theoretical Methods①
- Synthesis, Crystal Structure and Properties of a New Coordination Polymer Constructed with 3-(4-hydroxypyridinium-1-yl)phthalic Acid

