Characterization of phloroglucinol derivatives and diterpenes in Euphorbia ebracteolata Hayata by utilizing ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry
Cn-Jin Wng, Ying-Qio Jing, Dong-Hui Liu, Xio-Hong Yn,Shung-Cheng MA
aGuangzhou Institute for Drug Control, Guangzhou 510160, China
bGuangzhou University of Chinese Medicine, Guangzhou 510405, China
cNational Institute for Control of Pharmaceutical and Biological Products, Beijing 100050, China
1. Introduction
Euphorbia ebracteolata Hayata, a Chinese herb Langdu, has an effect of dissipating binds and insecticidal action.It is mainly used for treatment of scrofula, psoriasis [1]. The major constituents of E. ebracteolata include terpenoids, phloroglucinol derivatives,flavonoids, and sterols [2]. In the present study, we focus on the phloroglucinol derivatives and diterpenes. Diterpenes play an important role in the prevention of human diseases. The most prominent effects of the diterpenes in E. ebracteolata are antitumor, antibacteria and immune adjustment [3—5]. The structures of these phloroglucinol derivatives and diterpenes are summarized in Scheme 1. Conventional phytochemical approaches, which are time-consuming, have been used for studying the structures of phloroglucinol derivatives and diterpenes in E. ebracteolata [6—9]. To our knowledge, ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC—Q—TOF/MS), as a powerful hyphenated technique,has never been used to elucidate the structures of active compounds in E. ebracteolata.
In this study,we reported on the identification of phloroglucinol derivatives and diterpenes in the crude extract of E. ebracteolata by UPLC—Q—TOF/MS in positive-ion mode. Six reference chemicals, for example 2,4-dihydroxy-6-methoxy-3-methyl-acetophenone,were selected for characterization of the main components of E.ebracteolata for the first time.The mass spectral fragmentations of phloroglucinol derivatives and Jolkinolides abietane lactone in E. ebracteolata were discussed as well.
2. Materials and methods
2.1. Chemicals and materials
HPLC grade acetonitrile (Merck, Darmstadt, Germany), formic acid (CNW, Germany) were used, and ultra-pure water was prepared using a Milli-Q water purification system (Bedford,MA,USA).Ethanol(AR grade)for sample extraction was purchased from Guangzhou Chemical Reagent Factory (Guangzhou,China). All solvents were filtered through 0.22 μm membrane filters before analysis.
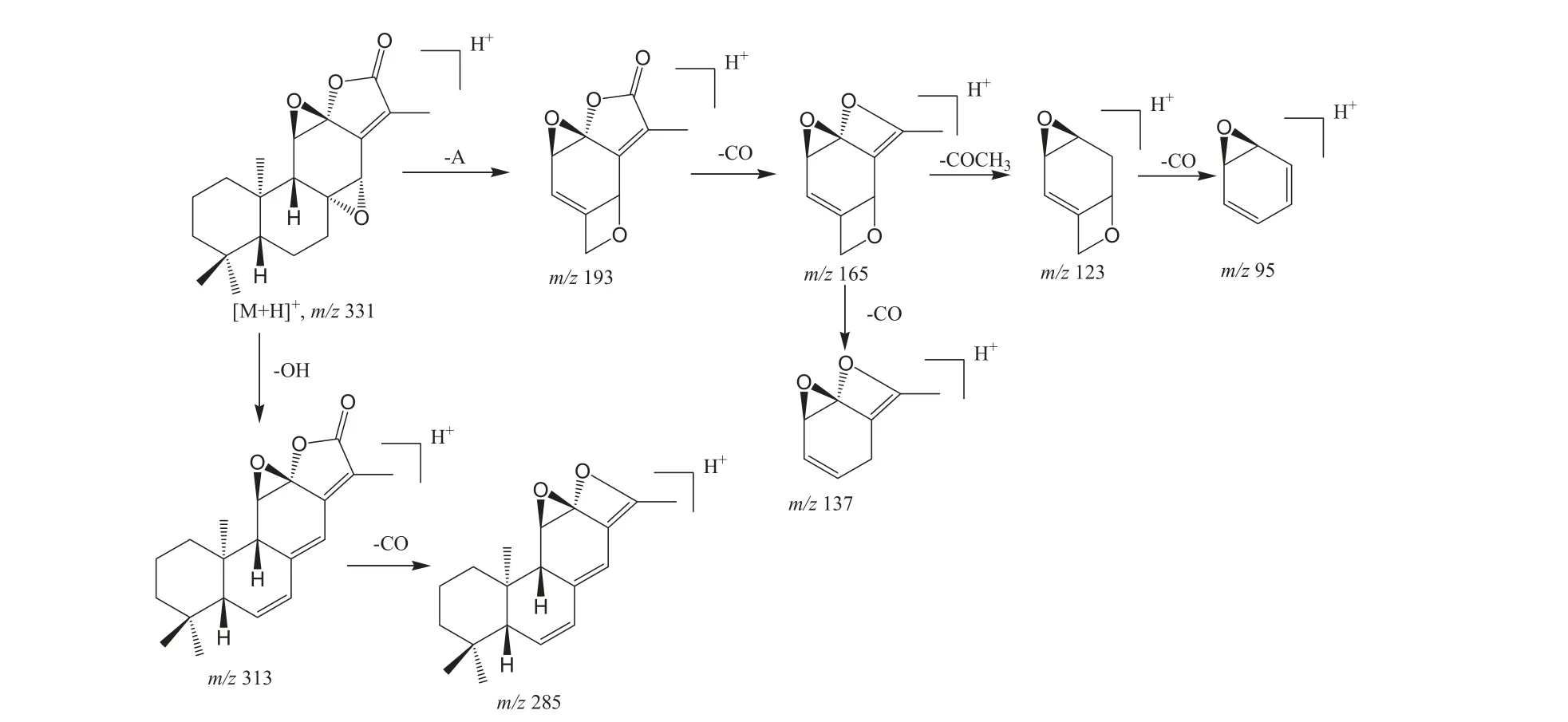
Scheme 1 A proposed fragmentation pathway of Jolkinolide B.
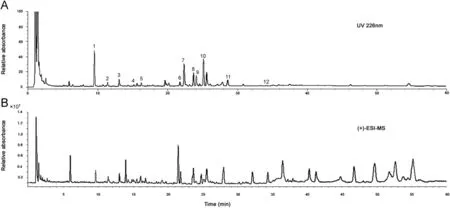
Fig.1 HPLC and ESI-MS chromatograms of E. ebracteolata: (A) HPLC chromatogram at 226 nm and (B) ESI-MS chromatogram in the positive mode.
?
The herbal medicine E. ebracteolata was obtained from Liaoning Institute for Food and Drug Control (China) and was identifi ed by chief pharmacist Boying Liu. A voucher specimen was deposited in Guangzhou Institute for Drug Control (Guangzhou, China).The reference standards of 2,4-dihydroxy-6-methoxy-3-methylacetophenone,2,2′,4,4′-tetrahydroxy-6,6′-dimethoxy-3,3′-dimethyl-7,5′-bisacetophenone, 3,3′-diacetyl-4,4′-dimethoxy-2,2′,6,6′-tetrahydroxydiphenylmethane, Jolkinolide A, Jolkinolide B and Yuexiandujisu D were obtained from Institute for Chinese Medicine and Natural Medicine, Pharmacy College, Jinan University (Guangzhou, China).
2.2. Sample preparation
The herbal materials were ground into a fi ne powder (through a sieve of 100 meshes). An aliquot of 1 g of E. ebracteolata,accurately weighed, was extracted for 30 min with 25 mL of ethanol on an ultrasonic water bath (30°C), and the extract was then fi ltered through a 0.22 μm syringe fi lter. The successive fi ltrate was used as the test solution.
2.3. Analytical method
Liquid chromatography was performed with an Agilent-1200 UPLC system (Agilent Corp., USA), equipped with a quaternary pump, UV detector, autosampler and column compartment.The column was an Agilent ZORBAX SB-C18 column(3.0 mm×100 mm, I.D., 5 μm). The mobile phase consisted of(A) 0.1% formic acid in water and (B) acetonitrile (ACN). The UPLC elution condition was optimized as follows: 30%—65% B(0—20 min),65%—75%B(20—30 min),75%—85%B(30—35 min),85% B (35—60 min) and isocratic at 30% B (60—63 min). The fl ow rate was at 0.4 mL/min. The column was maintained at 20°C, and the injection volume of reference compounds and samples was 2 μL. The analytes were monitored at 226 nm.
LC—MS detection was performed directly after UV measurements,which was performed by an Agilent 6538 UHD Accurate-Mass Q—TOF/MS (Agilent Corp., USA) equipped with an ESI interface. The TOF/MS analysis was performed using full scan mode and mass range was set at m/z 50—1700 in positive-ion mode. The conditions of ESI source were as follow: drying gas(N2) fl ow rate, 10 L/min; drying gas temperature, 350°C;Nebulizer,40 psig,capillary voltage,4000 V;fragmentor voltage,175 V; skimmer voltage, 65 V; octopole RF, 750 V. All the data were processed by Agilent MassHunter Software Ver. B. 03.01.Tuning mix was used for lock mass calibration in our assay.
3. Results and discussion
3.1. Optimization of HPLC and mass spectrometry conditions
To obtain desirable HPLC chromatograms, the procedure of sample preparation was optimized in selecting the extraction solvent. Three concentrations of aqueous ethanol, 50% (v/v in water), 70% and 95% were used for sample preparation. As no signifi cant difference was observed among the obtained chromatograms, we selected 95% aqueous ethanol as the extraction solvent for this study. Among the fi ve columns (Zorbax SB-C18,Dikma-C18, Ecosil-C18, Grace-C18 and Phenomenex-C18) that were tested for the separation of the sample, Zorbax SB-C18 column gave the best chromatographic resolution. 0.03% (v/v)formic acid was added to the mobile phase to improve the mass spectrometry ionization efficiency and enable symmetric peak shapes.The UV detection wavelength was set at 226 nm,at which most components can be detected sensitively. Both the positive and negative ion modes were tested for MS analysis.Most phloroglucinol derivatives and diterpenes showed much cleaner mass spectral background and higher sensitivity in the positive mode than in the negative mode. The HPLC chromatogram and LC/MS total ion current of E. ebracteolata extract are given in Fig.1.
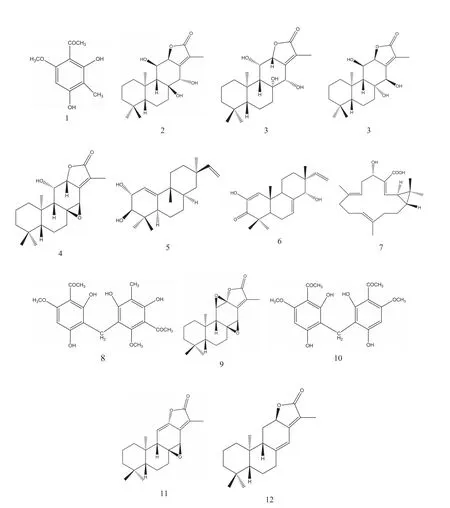
Fig.2 Chemical structures of identified compounds.
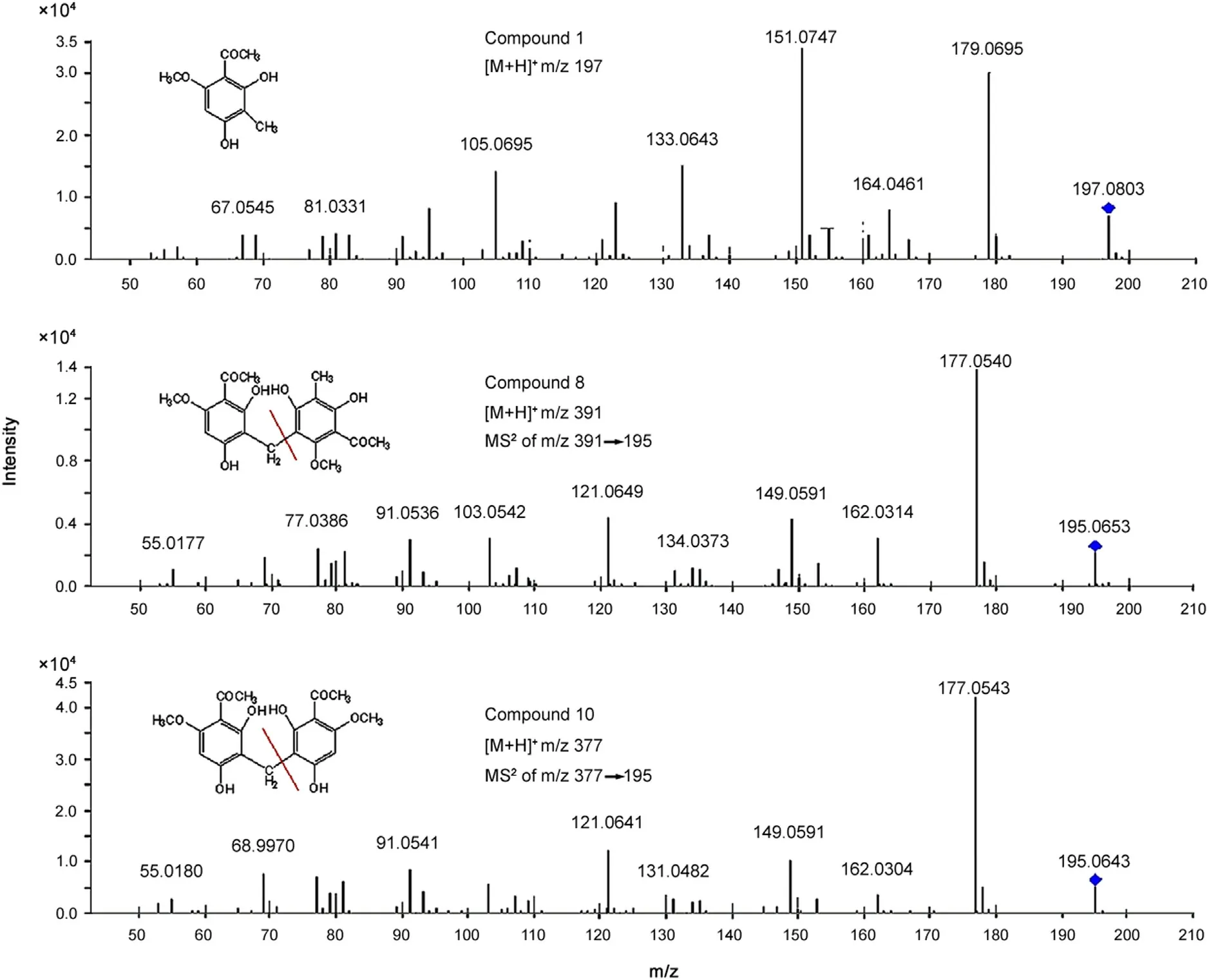
Fig.3 The MS2 spectra of compounds 1, 8 and 10.
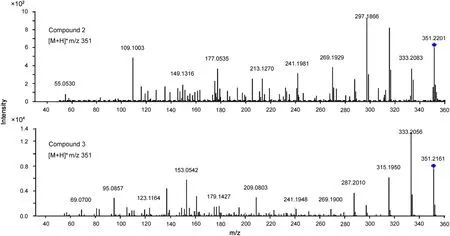
Fig.4 The MS2 spectra of compounds 2 and 3.
3.2. Rationale for the characterization of phloroglucinol derivatives and diterpenes
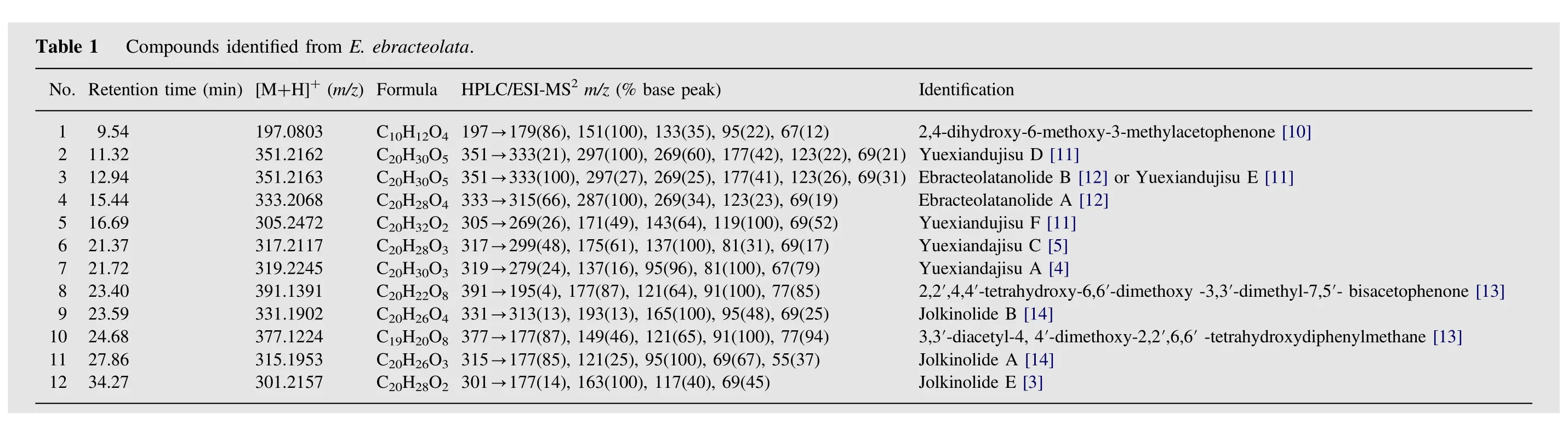
The identity of known compounds in the herbal extract was confirmed by comparing with reference standards according to the retention time and MS2spectra [15]. The unknown compounds were characterized by analyzing their fragmentation behaviors in MS2spectra and by referring to the available literature information. A total of 12 compounds were characterized (Table 1), six(1, 2, 8, 9, 10, and 11) of which were identified by comparing the mass spectra and retention times with those of reference standards.Jolkinolide E was reported from E. ebracteolata for the first time.The structures of confirmed compounds are shown in Fig.2.
3.3. Identification of phloroglucinol derivatives(compounds 1, 8 and 10)
Phloroglucinol derivatives were acetyl phenols containing hydroxyl,methoxy and acetyl groups. The mass spectral data of phloroglucinal derivatives showed a series of fragment ions apparently produced by gradual losses of hydroxyl, acetyl and methoxy, and five-membered-ring ion resulted from benzene ring cracking.
By comparing with mass spectra of a reference standard,compounds 1,8 and 10 were identified as 2,4-dihydroxy-6-methoxy-3-methylacetophenone,2,2′,4,4′-tetrahydroxy-6,6′-dimethoxy-3,3′-dimethyl-7,5′-bisacetophenone and 3,3′-diacetyl-4,4′-dimethoxy-2,2′,6,6′-tetrahydroxydiphenylmethane,respectively(Fig.3).
3.4. Identification of Jolkinolides type of ent-abietane lactone(compounds 2, 3, 4, 9, 11 and 12)
A number of Jolkinolides type of ent-abietane lactones have been reported from Euphorbia species[3].These lactones are tetracyclic diterpenoids, containing hydroxyl, epoxy groups. By comparison to reference standards, compounds 2, 9 and 11 were identified as Yuexiandujisu D, Jolkinolide B and Jolkinolide A, respectively.A proposed pathway of Jolkinolide B was given in Scheme 1.
Compound 3 gave [M+H]+at m/z 351. The MS/MS spectrum showed the same cleavage pattern as Yuexiandujisu D (Fig.4).Thus, compound 18 was tentatively identified as isomers of Yuexiandujisu D, named ebracteolatanolide B [12] or Yuexiandujisu E [11]. The MS/MS spectra of compounds 4 ([M+H]+at m/z 333)and 12([M+H]+at m/z 301),showing neutral losses of H2O and CO, were very similar to those of compounds 2, 9 and 11.Compounds 4 and 12 were tentatively identified as ebracteolatanolide A [12] and Jolkinolide E [3], respectively.
3.5. Identification of other free diterpenes(compounds 5,6 and 7)
Compounds 5 and 6 gave [M+H]+at m/z 305 and m/z 317,respectively. The MS/MS spectra of both compounds showed a similar fragmentation pathway with neutral losses of H2O and CO.Compounds 5 and 6 were tentatively identified as Yuexiandujisu F[11] and Yuexiandajisu C [5], respectively. Compound 7 gave[M+H]+at m/z 319.The MS/MS spectrum showed losses of H2O,CH3and COOH. Thus, compound 7 was tentatively identified as Yuexiandajisu A [4].
The work was supported by National Science and Technology Support Program and Drug Safety Critical Technology (2006BAI14B00)funding subject-Common and Important Drug Safety Standards(2006BAI14B01).
[1] B.Q. Yan, Y.Q. Zhang, Progress in Euphorbiae ebracteolate radix,J. Shandong Univ. Chin. Med. 32 (5) (2008) 432—435.
[2] X.W. Cao, W.S. Feng, The latest progress in studies of chemical components of Euphorbiae ebracteolate radix, J. Henan Univ. Chin.Med. 19 (115) (2004) 81—83.
[3] D.Y. Zhang, L.Y. Shi, Y.M. Wu, Progress in ent-abietane euphoriae diterpene lactones, Chin. J. Org. Chem. 29 (2) (2009) 188—196.
[4] Z.H. Xu, J. Sun, R.S. Xu, et al., Casbane diterpenoids from Euphorbia ebracteolata chemical studies of Lang-Du, a traditional Chinese medicine, Phytochemistry 49 (1) (1998) 149—151.
[5] Z.H. Xu, G.W. Qin, R.S. Xu, An isopimarane diterpene from Euphorbia ebracteolata Hayata, J. Asian Nat. Prod. Res. 2 (4)(2000) 257—261.
[6] G.M. Fu, B.Y. Yu, D.N. Zhu, Stuides on chemical constituents of Euphorbiae ebracteolata Hayata,J.China Pharm.Univ.34(4)(2003)377—379.
[7] H.M.Shi, Z.D.Min, Chemical constituents of Euphorbia ebracteolata Hayata, J. Chin. Pharm. Sci. 13 (3) (2004) 155—157.
[8] G.M. Fu, H.M. Qin, S.S. Yu, et al., Yuexiandajisu D, a novel 18-nor-rosane-type dimeric diterpenoid from Euphorbia ebracteolata Hayata, J. Asian Nat. Prod. Res. 8 (1—2) (2006) 29—34.
[9] G.M. Fu, B.Y. Yu, D.N. Zhu, Two novel phloroglucinol derivatives from Euphorbia ebracteolata Hayata,J.Asian Nat.Prod.Res.8(1—2)(2006) 149—153.
[10] Y.F.Dong,Y.M.Ding,Acylploroglucinal derivatives from the root of Euphorbia ebracteolata Hayata, J. Plant Resour. Environ. 1 (2)(1992) 1—3.
[11] H.M. Shi, I.D. Williams, H.H. Sung, et al., Cytotoxic diterpenoids from the roots of Euphorbia ebracteolata, Planta Med. 71 (4) (2005)349—354.
[12] W.X. Wang, X.B. Ding, Studies on diterpenoids from the roots of Euphorbia ebracteolata, Acta Pharm. Sin. 33 (2) (1998) 128—131.
[13] Z.Q. Yin, C.L. Fan, W.C. Ye, et al., Acetophenone derivatives and sesquiterpene from Euphorbia ebracteolata, Planta Med. 71 (10)(2005) 979—982.
[14] A.R. Lal, R.C. Cambie, P.S. Rutledge, et al., Ent-pimarane and entabietane diterpenes from Euphorbia fidjiana, Phytochemistry 29(1990) 2239—2246.
[15] M.Ye,W.Z.Yang,K.D.Liu,et al.,Characterization of flavonoids in Millettia nitida var. hirsutissima by HPLC/DAD/ESI-MSn, J. Pharm.Anal. 2 (1) (2012) 35—42.
 Journal of Pharmaceutical Analysis2013年4期
Journal of Pharmaceutical Analysis2013年4期
- Journal of Pharmaceutical Analysis的其它文章
- LC-MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats
- Application of analytical instruments in pharmaceutical analysis
- Investigation of the interaction between indigotin and two serum albumins by spectroscopic approaches
- In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia
- Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry
- Fingerprint analysis of Cirsium japonicum DC. using high performance liquid chromatography
