Fingerprint analysis of Cirsium japonicum DC. using high performance liquid chromatography
Hongli Ge, Muhetr Turhong, Munire Audkrem, Yuhi Tng,*
a School of Science,Xi'an JiaotongUniversity,Xi'an 710061,China
bKashi Teachers' College, Kashi 844006, China
1. Introduction
Cirsium japonicum DC, which belongs to the Compositae
family [1], is a wild perennial herb found in many areas of China as well as in Korea and Japan. It is listed in the Japanese and the Chinese Pharmacopoeia and has been used as an antihemorrhagic,anti-hypertension and an anti-hepatitis agent and also as a uretic in Chinese medicine. The main compounds of Cirsium japonicum DC are flavonoids, such as linarin, pectolinarin [2], acacetin, meletin, diosmetin [3],7-dihydroxy-6,4′-dimethoxyflavone [4] and hispidulin-7-neohesperido side [5]. Due to the growing conditions in the geographical origins, the quality and efficacy of Cirsium japonicum DC are somewhat different. In addition, people in some areas use Cirsium Setosum as Cirsium japonicum DC because of the same hemostatic activity.
Fingerprint technique is now widely used for the quality control of Chinese herb extracts and Chinese drug preparation and has been accepted as a useful tool to establish the Chinese medicine quality standard system [6-10]. Fingerprint analysis has been introduced and accepted by the World Health Organization (WHO) as an important means for assessing herbal medicines [11].
The present study aimed at developing the high performance liquid chromatography (HPLC) fingerprint of Cirsium japonicum DC so that the fingerprint model can accurately reflect the quality and guarantee clinical efficacy of Cirsium japonicum DC.
2. Experimental
2.1. Materials and reagents
Methanol of HPLC grade was from Fisher Scientific(NJ, USA). Glacial acetic acid was of analytical grade from China Medicine (Group) Shanghai Chemical Reagent Corporation (Shanghai, China). Deionized water was used throughout the study. Linarin (batch number: 20111023) was purchased from Jingke Biological Products Co. (Shanghai,China).The purity of linarin was determined to be higher than 98% by normalization of the peak areas detected by HPLC..
2.2. Plant materials
Commercial herb samples were collected from different areas in China, including the different orientation regions in China.Root of Cirsium japonicum DC was purchased from Zhejiang Province, Cirsium japonicum DC carbon and Cirsium setosum were bought in Shaanxi Province. The collected plant materials were dried at room temperature in the absence of light in a well-ventilated room. Professor Zengjun Guo authenticated the plant materials. Habitats and batch numbers of samples are shown in Table 1.
2.3. Instrumentation and conditions
HPLC analysis was carried out on a Shimadzu LC-10AT HPLC equipped with binary solvent delivery pump, and photodio dearray detector (PDA) connected to a Class-VP Software. The chromatographic separation was performed using a SHIM-PACK VP-ODS column (150 mm×4.6 mm i.d., 5 μm) maintained at 25°C. Detection wavelength was 337 nm with the sample injection volume of 10 μL; the flow rate was 0.8 mL/min. Water-glacial acetic acid (100:1, v/v)and methanol were used as mobile phase with a gradient elution. Total run time was 80 min. The gradient programmer was used according to the following profile: 0-5 min, 90%B linear decrease to 70%B; 5-60 min, linear decrease to 40%B;60-80 min, linear decrease to 20%B.
2.4. Preparation of standard solutions
In a clean, dry 50 mL volumetric flask,reference standard linarin was accurately weighed and dissolved in methanol to make a reference standard solution (204 μg/mL). The standard solution was stored at 4°C and brought to room temperature before use.
2.5. Sample preparation
The samples were ground into powder. 5.0 g of the powder was reflux extracted with 70% ethanol at 70°C for 2.5 h. After filtering through a 0.45 μm membrane filter, the extract was transferred into a 100 mL volumetric flask and adjusted to volume with 70% ethanol. The root of Cirsium japonicum DC, Cirsium japonicum DC carbon and Cirsium setosum were processed in the same way.
2.6. Method validation
The method validation mainly involved precision, reproducibility and stability.
Precision was determined by replicate injection of the same sample solution(no.13)for six times.Similarity of the 10 common chromatographic peaks was investigated with the software of Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine(Version 2004A).The similarity was over 0.99, which indicated a high precision.
Reproducibility was determined by injection of six individual sample solutions extracted from the same sample (no.13)in the same way. The similarity was calculated and exceeded 0.99, which indicated a good reproducibility.
Stability was tested with one sample solution stored at room temperature for 0,3,6,9,12 and 24 h;the similarity was calculated and exceeded 0.99,which indicated a good stability.
3. Results and discussion
3.1. Extraction procedure
Sample pre-treatment conditions(extraction methods,solvents and time) were optimized by investigating their effect on theextraction efficiencies for different classes of chemical markers used for HPLC fingerprints.
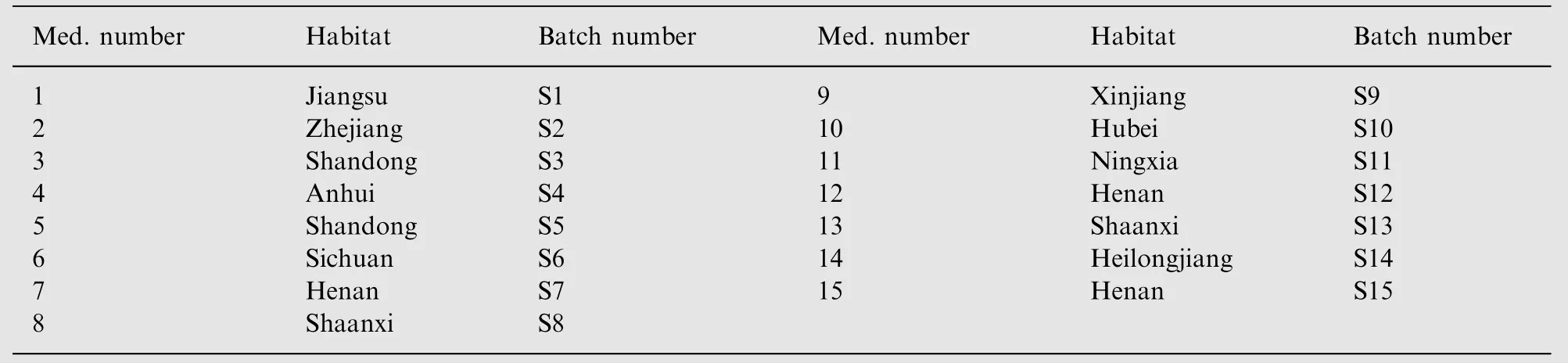
Table 1 Habitats and batch numbers of the samples.
Three extraction methods were investigated for extraction efficiencies(70%ethanol as the extraction solvent),including cold soak, reflux and ultrasonic extract. The HPLC peak areas of the compounds obtained from these three techniques were compared,and heat reflux was chosen because of high extraction efficiency.
Six extraction solvents were investigated for extraction efficiencies (heat reflux as the extraction method), including 45%, 70% methanol aqueous solution; methanol; and 45%,70%, 95% ethanol aqueous solution. 70% ethanol aqueous solution was selected as the extract solvent for extracting all compounds based on the HPLC results.
Extraction time under reflux was also investigated, and the results showed that most of the compounds were extracted within 2.5 h, and that longer period of reflux did not increase the contents significantly.
3.2. Optimization of HPLC conditions
Acetic acid was added to mobile phase B and suppress the tailing of the peaks of phenolic compounds. Concentration of 1% acetic acid aqueous solution was selected to ensure the reproducibility of the fingerprint chromatogram. Since isocratic elution was not suitable for the complete separation of the phenolic compounds (linarin and pectolinarin) in Cirsium japonicum DC, gradient elution was adopted. This gradient of the mobile phase could achieve maximum throughput and optimal resolution. The wavelength for detection of the components in Cirsium japonicum DC was selected by using a PDA detector.It was found that the chromatograms at 337 nm could well represent the profile of the constituents.
3.3. HPLC fingerprints and similarity analysis
With the optimal conditions, the fingerprints of Cirsium japonicum DC were established (Fig.1). It was found that these samples had similar HPLC profiles. Using the software of Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2004A), the representative standard fingerprint was generated by the median method (Fig.2).
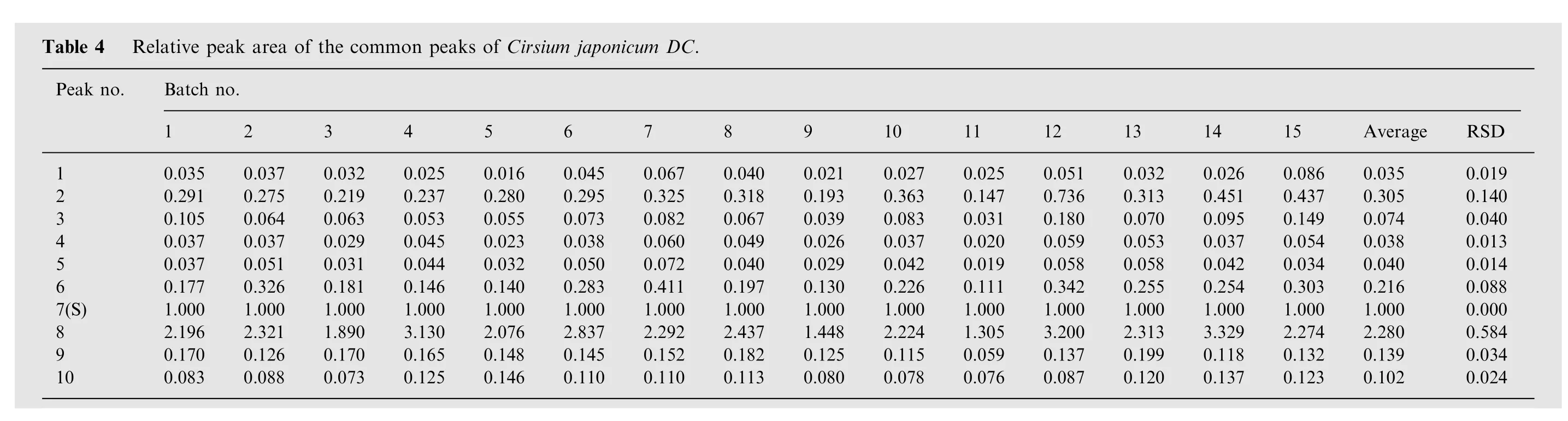
Peaks existed in the standard fingerprint with reasonable heights and good resolutions were assigned as ‘‘characteristic common peaks''.There were 10 characteristic peaks(from peak 1 to peak 10)in the chromatogram,which covered more than 90%of the total area. The similarity of Cirsium japonicum DC was calculated with the median method and time window width 0.5.The HPLC spectrum of Cirsium japonicum DC of Jiangsu (no.1)was considered as the reference spectrogram. These results(Table 2) indicated that the samples shared almost the same correlation coefficients of similarities, showing that the internal quality of these samples was excellent.The relative retention time and relative peak area of the common peaks were calculated(Tables 3 and 4), which indicated good similarities.
3.4. Overall qualitative and overall quantitative similarities of Cirsium japonicum DC
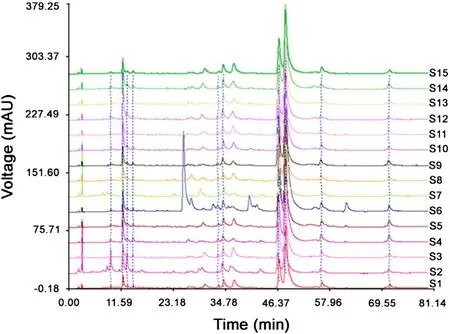
Fig.1 The fingerprints of 15 batches of Cirsium japonicum DC.S1-S15 represent the samples of no. 1-15.
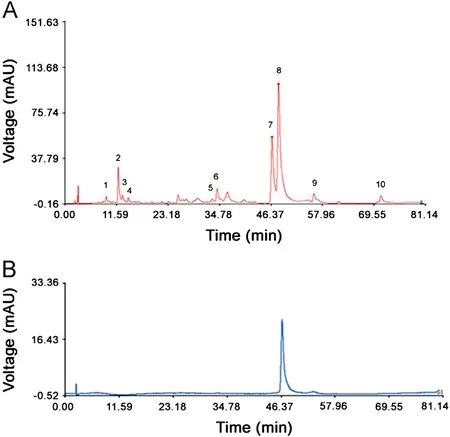
Fig.2 Standard fingerprint of Cirsium japonicum DC (A) and chromatogram of linarin (B). 10 peaks in (A) were assigned as‘‘characteristic common peaks''. Their retention time was 1 (9.295),2(12.003), 3 (12.946), 4 (14.228), 5 (33.132), 6 (34.259), 7 (46.606), 8(48.078), 9 (56.042), and 10 (71.095), respectively
Since the software of Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2004A) could not calculate the quantitative similarity, the quality control system of both the overall qualitative similarities and the overall quantitative similarities of traditional Chinese medicine chromatographic fingerprints[12]was used.The results of overall qualitative and overall quantitative similarities are shown in Table 5.

?

?
?

?
The overall qualitative similarities in which the similarity of cosine of angle SFwas combined with the qualitative similarity of peak area ratio S′Fwere proposed to accurately solve the qualitative assessment problem of chromatographic fingerprints.The percentage of module lengths W% was combined with the apparent quantitative similarity R%, the quantitative similarity calculated by vector shadow C% combined with the quantitative similarity P%, the content similarity Q% combined with the average percentage of mass M%, and the correction content similarity QF% combined with the average correction percentage of mass MF%,respectively,to compose the first,second,third and fourth overall quantitative similarities.During the evaluation,that both SFand S′Fwere more than 0.9 was the required condition and any one of the above four pairs of quantitative similarities could be selected to assess the characteristics of component contents, in which the pair mentioned above should be within 90%-110%for the prescribed dosages,and 85%-120%for crude herbal drugs, respectively, and the differences should be less than 10% between each pair. If the results meet the requirements, the samples can be regarded as qualified.
3.5. Comparison of two similarity evaluation methods
From Tables 2-4, we could find that all samples had good similarities, which were calculated with the software of Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2004A).This system could make qualitative identification of the samples.
From Table 5, we could find that all samples were qualified with the third and the fourth overall quantitative similarities,while the samples of no.4,7,and 15 were not qualified with the first and the second overall quantitative similarities. This method could simultaneously determine the contributions of big fingerprints and small fingerprints to the system, and had the function of qualitative and quantitative identification.
3.6. Comparison of Cirsium japonicum DC, Cirsium
japonicum DC root,Cirsium japonicum DC carbon and Cirsium setosum
The HPLC spectrum of Cirsium japonicum DC,Cirsium japonicum DC root,Cirsium japonicum DC carbon,and Cirsium setosum was compared (Fig.3). It was found that the spectrum of Cirsium japonicum DC root and Cirsium japonicum DC carbon somewhat differed from that of Cirsium japonicum DC in peak retention time and peak relative area. Cirsium japonicum DC root and Cirsium japonicum DC carbon had more small polar substances;moreover,it was reported that Cirsium japonicum DC carbon had more effective hemostasis [13], which indicated that the hemostasis activity may be related to these small polar substances.
Due to the same hemostasis activity, sometimes Cirsium Setosum is used as Cirsium japonicum DC. It was found that their HPLC spectrum had many differences and that the similarity was less than 0.5,which indicated that they were two totally different plants.
4. Conclusion
An HPLC method was established for fingerprint analysis of Cirsium japonicum DC.This method showed high precision and good repeatability, and provided the basis for the quality control of Cirsium japonicum DC. The fingerprints of Cirsium japonicum DC were obtained and analyzed by the software Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2004A) and the quality control system of both the overall qualitative similarities and the overall quantitative similarities of traditional Chinese medicine chromatographic fingerprints, which reflected the quality from different sides.

Fig.3 Comparisons between Cirsium japonicum DC and Cirsium japonicum DC root (A), Cirsium japonicum DC and Cirsium japonicum DC carbon (B) and CirsiXAum japonicum D and Cirsium setosum (C).
The work was supported by the Main Program of Autonomous Region College Scientific Research Program (XJEDU2008145).
[1] The State Pharmacopoeia Committee of the People's Republic of China. Pharmacopoeia of the People's Republic of China,Chemical Industry Press, Beijing, 2010, p. 23.
[2] J.C.Park,J.H.Lee,J.S.Choi,A flavone diglycoside from Cirsium japonicum var1 ussu riense,Phytochemistry 39(1)(1995)261-262.
[3] X.L. Jiang, C.L. Fan, W.C. Ye, Studies on chemical component of Cirsium japonicum DC., J. Chin. Traditional Herbal Drugs 37(4) (2006) 510-514.
[4] Y.C. Gu, A.A. Tu, Studies on chemical component of Cirsium japonicum DC.,J.Zhong Guo Zhong Yi Yao Za Zhi 17(8)(1992)4892-4901.
[5] H.Ishida,T.Umino,K.Tsuji,et al.,Studies on antihemorrhagic substances in herbs classified as hemostatics in Chinese medicine:VII On the antihemorrhagic principle in Cirsium japonicum DC., Chem. Pharm. Bull 35 (2) (1987) 861-864.
[6] L.J.Zhang,Application of the chromatographic fingerprint in the quality standard system of Chinese medicine, J. Anhui Med.Pharm. 13 (11) (2009) 1414-1417.
[7] L. Zhao, C. Huang, Z. Shan, et al., Fingerprint analysis of Psoralea corylifolia L. by HPLC and LC-MS, J. Chromatogr. B 821 (2005) 67-74.
[8] M. Gu, F. Ouyang, Z. Su, Comparison of high-speed countercurrent chromatography and high-performance liquid chromatography on fingerprinting of Chinese traditional medicine,J. Chromatogr. A 1022 (2004) 139-144.
[9] Y.-B. Ji, G. Alaerts, C.-J. Xu, et al., Sequential uniform designs for fingerprints development of Ginkgo biloba extracts by capillary electrophoresis, J. Chromatogr. A 1128 (2006) 273-281.
[10] G. Alaerts, N. Matthijs, J. Smeyers-Verbeke, et al., Chromatographic fingerprint development for herbal extracts: a screening and optimization methodology on monolithic columns, J. Chromatogr. A 1172 (2007) 1-8.
[11] World Health Organization. Guidelines for the Assessment of Herbal Medicines. WHO, Geneva, 2000, p. 1.
[12] G.X.Sun,Y.Song,Y.M.Bi,et al.,Quality control system of overall qualitative similarities and overall quantitative similarities of chromatographic fingerprints,J.Central South Pharm.5(3)(2007) 263-267.
[13] Q.F.Gong,L.Fu, The comparison of TLC and HPLC spectrum between Cirsium japonicum DC. and Cirsium japonicum DC.Carbon, in: Conference Proceedings of the Fourth Conference in Traditional Chinese Medicine Proceeding of Association of Chinese Medicine. Nanchang, Association of Chinese Medicine,2004, pp. 119-120.
 Journal of Pharmaceutical Analysis2013年4期
Journal of Pharmaceutical Analysis2013年4期
- Journal of Pharmaceutical Analysis的其它文章
- LC-MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats
- Characterization of phloroglucinol derivatives and diterpenes in Euphorbia ebracteolata Hayata by utilizing ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry
- Application of analytical instruments in pharmaceutical analysis
- Investigation of the interaction between indigotin and two serum albumins by spectroscopic approaches
- In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia
- Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry
