四溴代對(duì)苯二甲酸根及二吡啶基丙烷構(gòu)筑的鋅(Ⅱ)及鎘(Ⅱ)配位聚合物的合成及晶體結(jié)構(gòu)
呂旭燕 劉建鋒 劉 艷 高玲玲 肖立群 胡拖平
(中北大學(xué)理學(xué)院化學(xué)系,太原 030051)
At present, owing to the intriguing topological architectures and extensive applications[1-6], such as magnetism[7],porosity[8-9],non-linear optical behavior[10-12],adsorption, separation, catalysis etc., the investigation of rational design and synthesis of the metal-organic frameworks (MOFs) has become one of the noteworthy themes[13-16].
As is known, the structures of the complexes depend on organic ligands, metal ions and synthetic methods[17-25], etc., so the choice of appropriate metal ions and the organic ligands with suitable functional groups (such as carboxyl group and pyridyl) is very important in the designing and synthesizing of the complexes.
Due to the 2,3,5,6-tetrabromoterephthalic acid(H2TBTA) ligand displays various coordinated modes,it can construct many novel topological architectures.Meanwhile, the obvious spatial effects of the bromine substituents of the aromatic ring have significant effects to the spatial orientation of constructing MOFs.Besides, the introduction of the auxiliary ligand 1,3-bis (4-pyridyl)propane (BPP) may affect the architectures of MOFs. In the BPP, the two pyridyl groups connected by a flexible aliphatic chain makes the distance of the both pyridyl groups changeable,hence, the BPP possesses rich coordinated modes to produce novel structures.
Up to now, only several complexes with H2TBTA and transition metals (Co, Ni, Cu, Zn, Cd, Pb, Ag,Mn)[26-28]were reported. In this context, we report the synthesis, crystal structures of two new coordination polymers constructed by H2TBTA and BPP ligands,namely, [Zn(TBTA)(BPP)]n(1) and [Cd(TBTA)(BPP)2(H2O)2]n(2). In complex 1, tetrahedral Zn centers were joined into a 2D wave-like layer by TBTA2-anions and BPP ligands. Octahedral Cd centers were bridged into a 1D chain through TBTA2-anions, however, BPP molecules only act as capping ligands.
1 Experimental
1.1 Materials and methods
All reagents and solvents were commercially available and used without furher purification. The structures of the complexes were determined on a Bruker Apex ⅡCCD diffractometer and solved by direct methods using the SHELXTL program.Elemental analyses were performed by a Vario EL analyzer. The IR spectra (KBr pellets) were recorded on a FTIR-8400S spectrometer in the range of 4 000~400 cm-1. The thermogravimetric analyses were carried out on a ZCT-A analyzer at the heating rate of 10 ℃·min-1under air atmosphere.
1.2 Synthesis of [Zn(TBTA)(BPP)]n(1)
A 5 mL distilled water solution of H2TBTA (10 mg, 0.02 mmol) and BPP (10 mg, 0.05 mmol) was placed at the bottom of a straight glass tube, then CH3OH/H2O solution (2 mL, 1∶1, V/V) was dropwise added. At last, a 5 mL methanol solution containing Zn(NO3)2·6H2O (20 mg, 0.067 mmol) was carefully added onto it. The tube was sealed and stood at room temperature. The colorless crystal suitable for X-ray analysis was obtained upon diffusion of the solvents after ten days (Yield: 66%). Anal. Calcd.(%) for Zn C21H14Br4N2O4(743.35): C, 33.931; H, 1.898; N,3.768. Found (%): C, 34.003; H, 1.721; N, 3.752. IR(KBr, cm-1): 2 927 (m), 1 616 (vs), 1 510 (m), 1 396(s), 1 297 (s), 1 230 (m), 1 063 (m), 1 037 (m), 817(m), 698 (w), 571 (m), 524 (w).
1.3 Synthesis of [Cd(TBTA)(BPP)2(H2O)2]n(2)
As the same synthetic procedure of the complex 1, the complex 2 was synthesized except that the metal salt was Cd(NO3)2·4H2O (20 mg, 0.065 mmol).The colorless block crystal (Yield: 71%) was generated after a period of one week. Anal. Calcd. (% ) for C34H32Br4CdN4O6(Mr=1 024.68): C, 39.854; H, 3.148;N, 5.468. Found(%): C, 39.74; H, 3.178; N, 5.41.IR (KBr, cm-1): 3 207 (m), 2 934 (m), 1 609 (vs), 1 503 (w), 1 390 (s), 1 317 (s), 1 217 (m), 1 077 (w), 1 010 (m), 817 (m), 677 (w), 558 (m), 511 (w).
1.4 X-ray crystallography
The suitable single crystals with dimensions of 0.19 mm×0.15 mm×0.10 mm for the complex 1, 0.15 mm ×0.10 mm ×0.10 mm for the complex 2 were determined on a Bruker Apex ⅡCCD diffractometer.Data were collected at 296.0 K by using Mo Kα radiation (λ=0.071073 nm). The structures were solved by direct method using SHELXS-97 program[29]. All of the non-hydrogen atoms were located with successive difference Fourier syntheses and refined by full-matrix least-squares methods on F2of SHELXL-97 program[30]with anisotropic thermal parameters. The hydrogen atoms were added theoretically. Crystallographic data and structural refinement summary for complexes are listed in Table 1. The selected bond lengths and bond angles are listed in Table 2.
CCDC: 854381, 1; 854380, 2.
2 Results and discussion
2.1 Crystal structures of the complexes
2.1.1 [Zn(TBTA)(BPP)]n(1)
Single-crystal X-ray diffraction analysis reveals that the complexe 1 is monoclinic crystal system with
P21/n space group. The asymmetric unit is composed of Zn(Ⅱ)ion, TBTA2-anions and BPP molecule (Fig.1).As shown in Fig.1, the Zn(Ⅱ)ion is four-coordinated and the coordinated atoms around it are two oxygen atoms supported by two TBTA2-anions, two nitrogen atoms from two BPP ligands. The uncoordinated oxygen atoms from carboxyl groups of H2TBTA ligands are deprotonated and provide the hydrogen bonding acceptors to form strong H-bonding interactions with the hydrogen atoms from BPP ligands (Fig.1). The Hbonding interactions make the structure more stable.The details of the H-bonding interactions are listed in Table 3.
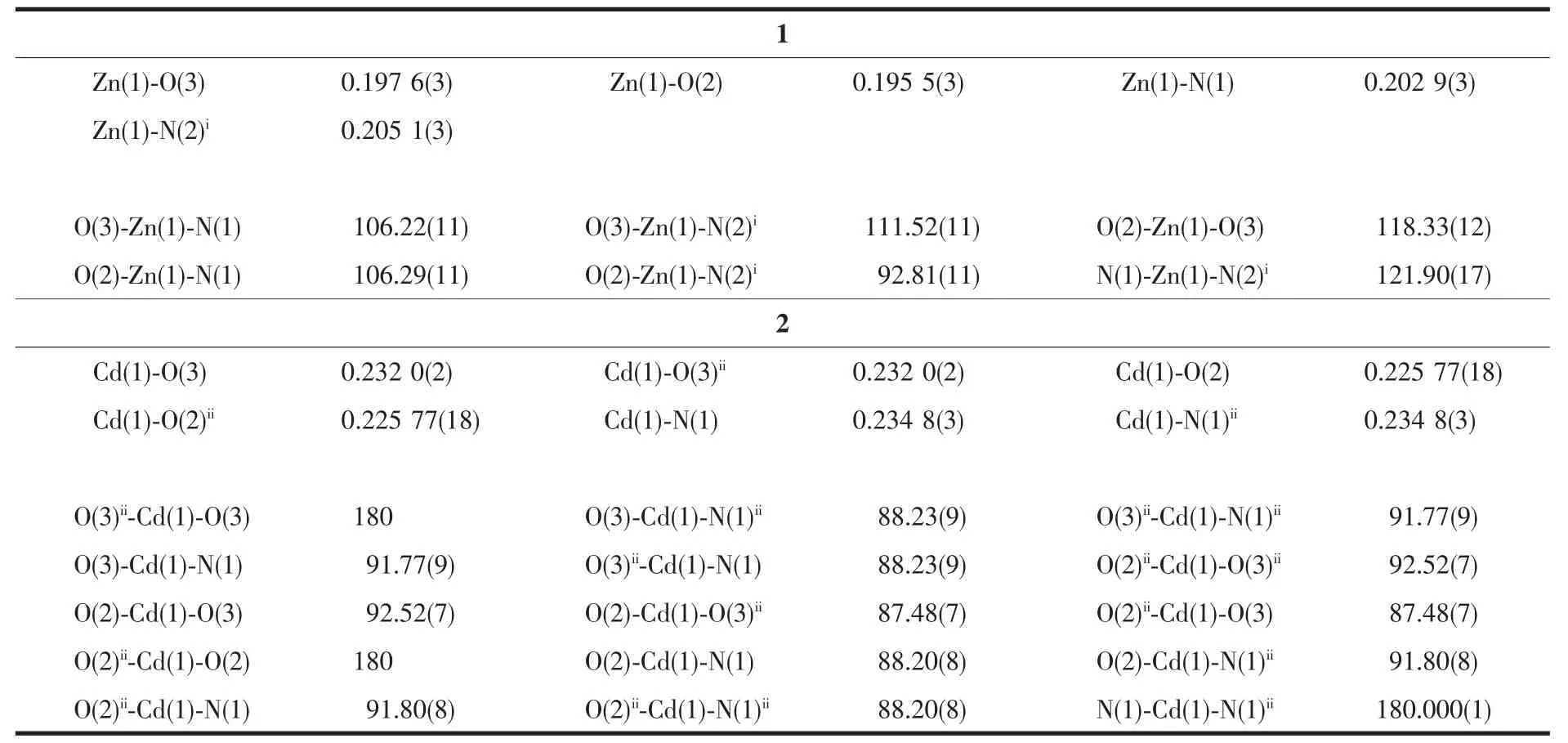
Table 2 Selected bond lengths (nm) and bond angles (°) for complex 1 and 2
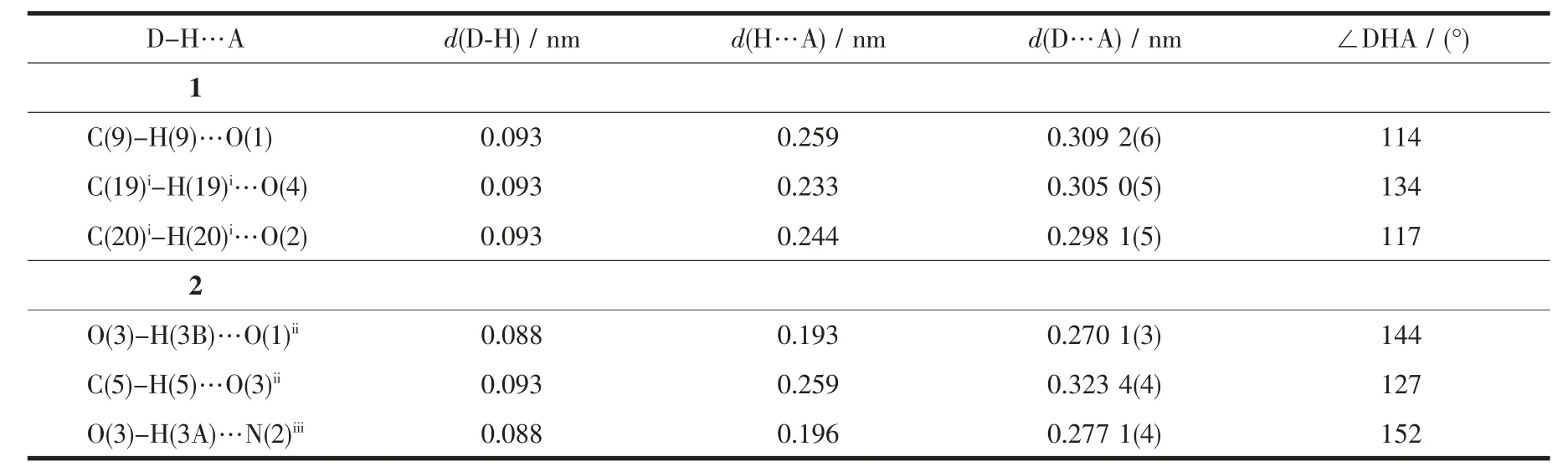
Table 3 Hydrogen bond lengths and bond angles for complexes 1 and 2

Fig.1 Coordination environment and hydrogen bonds of the complex 1 with thermal ellipsoids at 50%probability

Fig.2 Zn-carboxylate 1D zigzag chain of the complex 1

Fig.3 View of 2D wave-like layer bridged by BPP and TBTA2-of the complex 1
In the complex 1, the carboxyl groups of H2TBTA ligands adopt monodentate mode. Thus, the structure infinitely extends to be a zigzag chain by TBTA2-anions with the same distances of Zn(1)…Zn(1)ivand Zn(1)…Zn(1)v, 1.094 5 nm (Fig.2). Then the zigzag chains are further bridged by BPP ligands to generate a 2D wave-like layer with the same distances of Zn(1)…Zn(1)iand Zn(1)…Zn(1)vi, 1.087 2 nm (Fig.3). In order to clearly view the structure, the topological ananlysis was used. Taken the Zn (Ⅱ)ions as fourconnected nodes, TBTA2-anions and BPP molecules as linear connectors, the 2D wave-like structure can be simplified as a (4,4) net framework, and the Schlafli symbol is {4^4.6^2}. The interpenetration of two sheets is topologically identical to that shown in Fig.4.
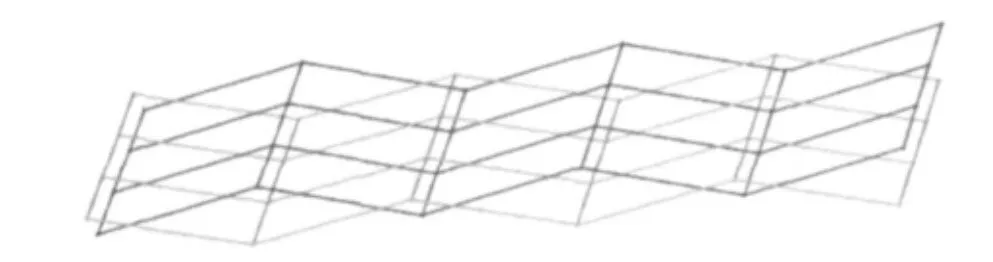
Fig.4 Two-fold interpenetration of (4,4) net framework of the complex 1
2.1.2 [Cd(TBTA)(BPP)2(H2O)2]n(2)
Single-crystal X-ray diffraction analysis shows that the complex 2 is triclinic crystal system withspace group. The Cd (Ⅱ)ion is six-coordinated and surrounded by two oxygen atoms from two TBTA2-anions, two nitrogen atoms from two BPP ligands and two oxygen atoms from two coordinated H2O molecules(Fig.5). Among these atoms, the four oxygen atoms locate at the equatorial plane and the two nitrogen atoms are in axial. Because of the spatial effects of the bromine substituents, the planes of aromatic ring of the TBTA2-anions and the BPP ligands are nearly vertical (dihedral angle is 94.416°). Each TBTA2-anion bridges two adjacent metal ions (Cd(1)…Cd(1)ii=1.170 8 nm, Cd(1)…Cd(1)vii=1.170 8 nm)) to give rise to a 1D chain (Fig.6).
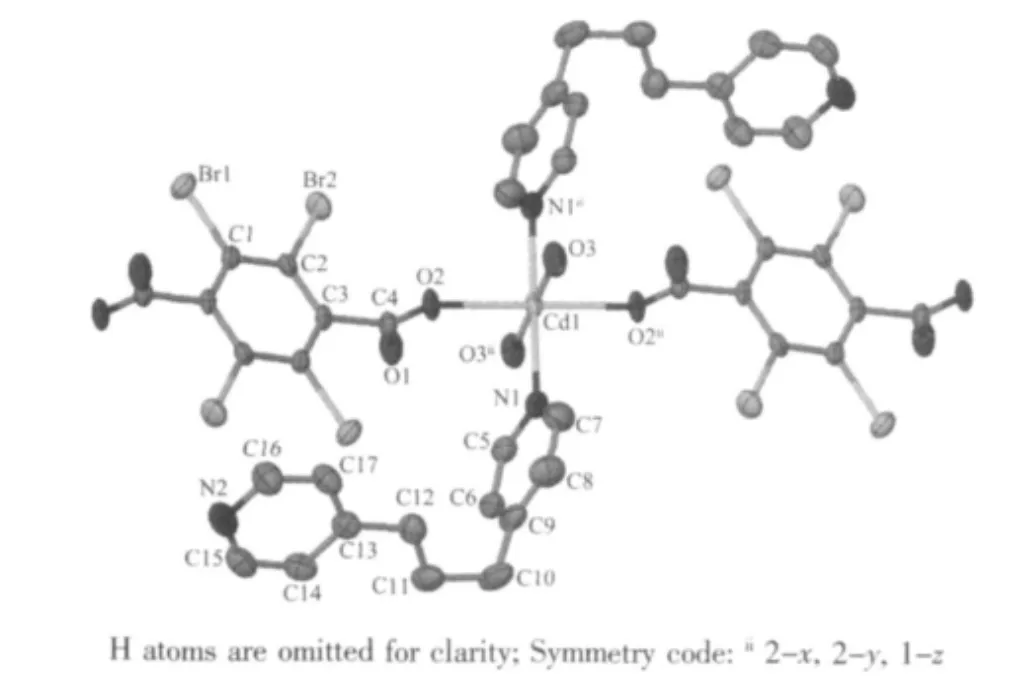
Fig.5 Coordination environment of Cd (Ⅱ)with thermal ellipsoids at 50% probability
As the complex 1, the oxygen atoms of carboxyl groups in 2 are deprotonated and provide the hydrogen bonding acceptors. So, the uncoordinated oxygen atoms of carboxyl groups form intramolecular H-bonding interactions with the hydrogen atoms of the coordinated H2O molecules (Table 3, Fig.6). The Hbonding interactions reinforce the stability of the 1D chain. There also exist intermolecular H-bonding interactions among the 1D chains (Table 3, Fig.7).
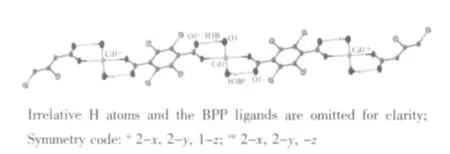
Fig.6 1D chain and hydrogen-bondings of the complex 2 with thermal ellipsoids at 50% probability
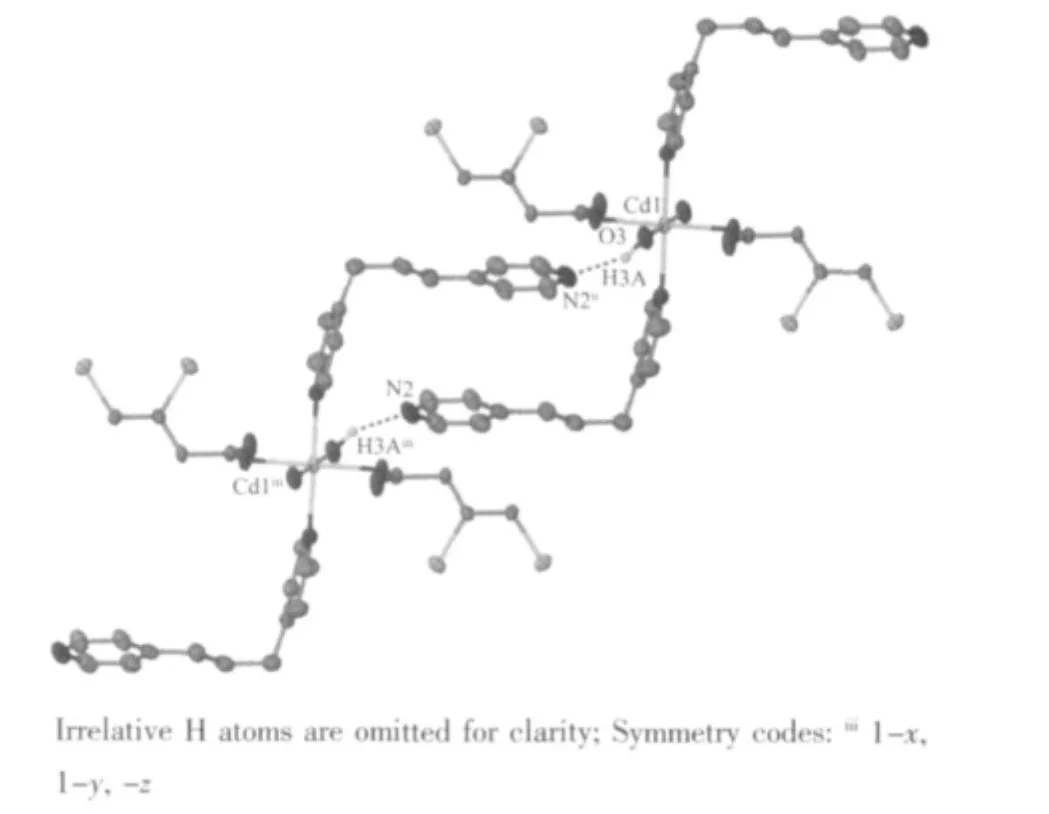
Fig.7 View of the intermolecular H-bonding interactions of the complex 2 with thermal ellipsoids at 50%probability
2.2 IR spectra of the complexes
In the complex 1, the absence of characteristic band at 3 300 cm-1reveals no H2O molecule exists.While the absorption band at 3 207 cm-1in complex 2 means the O-H characteristic stretching vibrations of H2O corresponding to the known structure. The IR spectra of the complexes exhibit the typical antisymmetric (1 616 cm-1in 1, 1 609 cm-1in 2) and symmetric (1 396 cm-1in 1, 1 390 cm-1in 2)stretching bands of carboxyl groups[31], the values Δν(νas(COO-)-νs(COO-)) of 1 (220 cm-1) and 2 (219 cm-1) indicate that TBTA2-is monodentate[32-33]. Meanwhile, characteristic bands nearby 1 530 cm-1(1 510 cm-1in 1, 1 503 cm-1in 2) belong to the stretching vibration of -N=C- of BPP ligands. The results of the IR spectra analyses of the complexes are in consistent with that of single-crystal X-ray analyses.
2.3 Thermogravimetric analyses
The thermal stabilities of the complexes are investigated by thermogravimetric analyses (TGA)technique and the TGA curves are in Fig.8 and Fig.9.
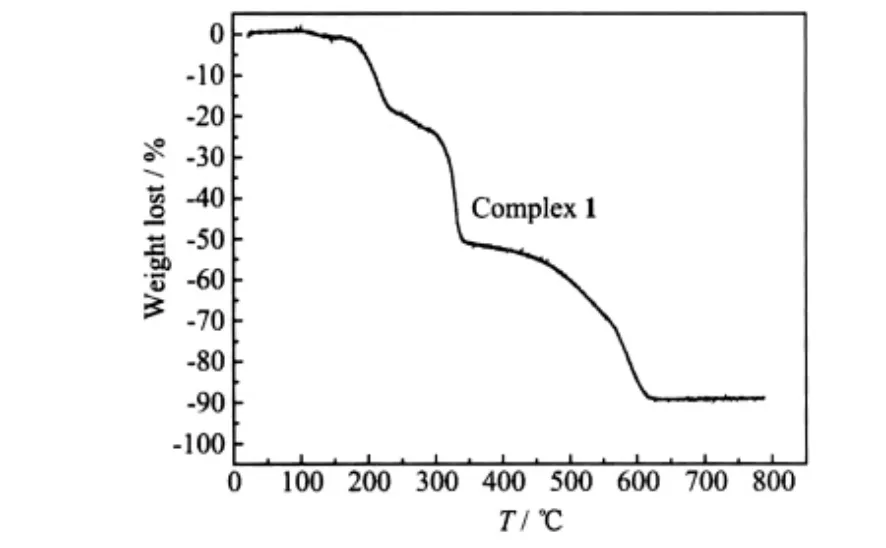
Fig.8 Thermogravimetric analysis for the complex 1
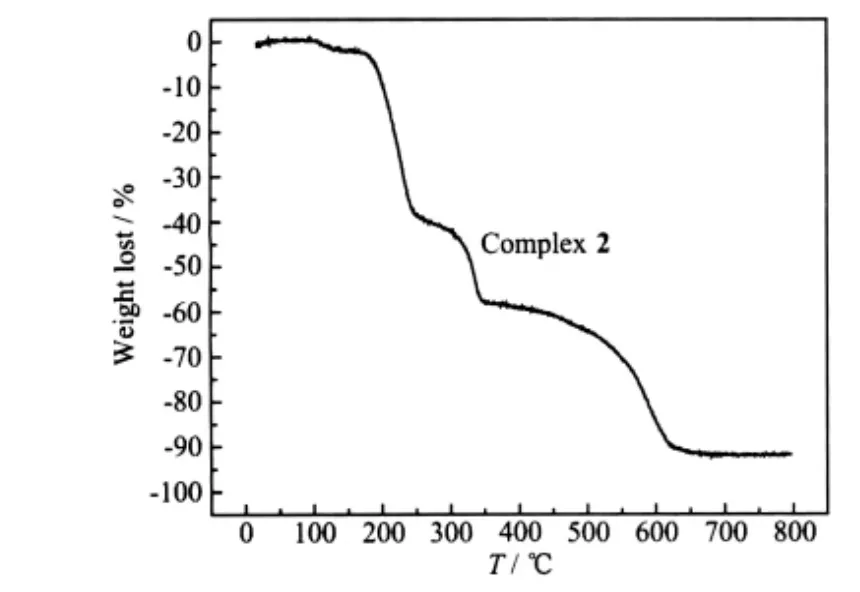
Fig.9 Thermogravimetric analysis for the complex 2
The curve of complex 1 reveals that the complex is stable before 170 ℃. The weight loss of 25.29%from 170 to 307 ℃corresponds to the remove of BPP molecules (Calcd. 26.67%). Then the complex begins to disintegrate with the growing temperature. There is no weight loss after 622 ℃. The final product is ZnO(Obsd. 10.93%, Calcd. 10.95%).
For complex 2, the initial weight loss of 2.81%from 105 ℃to 131 ℃belongs to the loss of two coordinated H2O molecules (Calcd. 3.52% ). The second total weight loss of 41.88% at 298 ℃(Calcd.42.23%) dues to the coordinated BPP ligands. Then the complexe starts to disintegrate. No weight loss can be observed after 618 ℃. The final product is CdO(Obsd. 10.87%, Calcd. 12.52%).
Reference:
[1] Surblé S,Serre C,Mellot-Draznieks C,et al. Chem.Commun.,2006,6:284-286
[2] Yaghi O M, O′Keeffe M, Ockwig N W, et al. Nature, 2003,423(6941):705-714
[3] Huang Y G, Wu B L, Yuan D Q, et al. Inorg. Chem., 2007,46(4):1171-1176
[4] Wu C D, Hu A, Zhang L, et al. J. Am. Chem. Soc., 2005,127(25):8940-8941
[5] Gao X M, Li D S, Wang J J, et al. Cryst. Eng. Commun.,2008,10:479-482
[6] Zhang J P, Chen X M. J. Am. Chem. Soc., 2008,130:6010-6017
[7] Kahn O. Acc. Chem. Res., 2000,33(10):647-657
[8] Kesanli B, Lin W. Coord. Chem. Rev., 2003,246:305-326
[9] Kitagawa S, Kitaura R, Noro S. Angew. Chem. Int. Ed.,2004,43(18):2334-2375
[10]Le Bozec H, Le Bouder T, Maury O, et al. J. Opt. A. P: Pure Appl. Opt., 2002,4:S189-S196
[11]Maury O, Le Bozec H. Acc. Chem. Res., 2005,38(9):691-704
[12]Evans O R, W Lin. Acc. Chem. Res., 2002,35(7):511-522
[13]Liu Y L, Eubank J F, Cairns A J, et al. Angew. Chem. Int.Ed., 2007,46:3278-3283
[14]Maspoch D, Ruiz-Molina D, Veciana J. Chem. Soc. Rev.,2007,36:770-818
[15]Férey G. Chem. Soc. Rev., 2008,37(1):191-214
[16]Kitagawa S, Matsuda R. Coord. Chem. Rev., 2007,251:2490-2509
[17]Hao H Q, Liu W T, Lin Z J, et al. CrystEngComm., 2009,11:967-971
[18]Ye B H, Tong M L, Chen X M. Coord. Chem. Rev., 2005,249(5-6):545-565
[19]Huang Y G, Yuan D Q, Pan L, et al. Inorg. Chem., 2007,46:9609-9615
[20]Hu S, Chen J C, Tong M L, et al. Angew. Chem. Int. Ed.,2005,44:5471-5475
[21]An H Y, Wang E B, Xiao D R, et al. Angew. Chem. Int.Ed., 2006,45:904-908
[22]Sun D F, Collins D J, Ke Y X, et al. Chem.-Eur. J., 2006,12:3768-3776
[23]Brammer L. Chem. Soc. Rev., 2004,33:476-489
[24]Li X L, Chen K, Liu Y, et al. Angew. Chem, Int. Ed., 2007,46(36):6820-6823
[25]Du M, Li C P, Zhao X J. Cryst. Growth Des., 2006,6(1):335-341
[26]Li C P, Chen J, Du M. CrystEngComm., 2010,12:4392-4402
[27]Li C P, Tian Y L, Guo Y M. Polyhedron, 2009,28:505-510
[28]JIN Mei-Fang(靳梅芳), DAI Fang-Na(戴昉納), SUN Dao-Feng(孫 道 峰). Chinese J. Struct. Chem.(Jiegou Huaxue),2010,29(12):1834-1840
[29]Sheldrick G M. SHELXS97, Program for the Solution of Crystal Structure, Germany: University of Gottingen, 1997.
[30]Sheldrick G M. SHELXL97, Program for the Refinement of Crystal Structure, Germany: University of Gottingen, 1997.
[31]Marinho M V, Yoshida M I, Guedes K J, et al. Inorg.Chem., 2004,43(4):1539-1544
[32]Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. 5th Ed. New York: Wiley Interscience, 1997.
[33]Miao M M, Liao D Z, Jiang Z H, et al. Transition Met.Chem., 1997,22(1):39-41

