Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
LIU Gongping (劉公平), HOU Dan (侯丹), WEI Wang (衛(wèi)旺), XIANGLI Fenjuan (相里粉娟) and JIN Wanqin (金萬勤)
?
Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
LIU Gongping (劉公平), HOU Dan (侯丹), WEI Wang (衛(wèi)旺), XIANGLI Fenjuan (相里粉娟) and JIN Wanqin (金萬勤)**
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
Pervaporation has attracted considerable interest owing to its potential application in recovering biobutanol from biomass acetone-butanol-ethanol (ABE) fermentation broth. In this study, butanol was recovered from its aqueous solution using a polydimethylsiloxane (PDMS)/ceramic composite pervaporation membrane. The effects of operating temperature, feed concentration, feed flow rate and operating time on the membrane pervaporation performance were investigated. It was found that with the increase of temperature or butanol concentration in the feed, the total flux through the membrane increased while the separation factor decreased slightly. As the feed flow rate increased, the total flux increased gradually while the separation factor changed little. At 40°C and 1% (by mass) butanol in the feed, the total flux and separation factor of the membrane reached 457.4 g·m-2·h-1and 26.1, respectively. The membrane with high flux is suitable for recovering butanol from ABE fermentation broth.
pervaporation, butanol, PDMS, ceramic, composite membrane
1 INTRODUCTION
In recent years, with the increase of petroleum price and shortage of petroleum resource, the utilization of renewable biomass to produce biofuel is increasing. Although the widely used biofuel is ethanol, it is found that butanol, as a new liquid fuel, has more superior properties, such as higher energy content, easy to transport, less evaporation, direct use without modification to the engine of the car [1]. Therefore, production of butanol has received more and more attention.
Acetone-butanol-ethanol (ABE) fermentation is one of the largest biotechnological processes to product butanol. The scale of butanol fermentation is only second to that of ethanol [2]. However, the fermentation process suffers from severe inhibition due to the high toxicity of butanol to microorganisms even at low concentrations (4-6 g·L-1). The microorganisms stop growth completely at the butanol concentration of approximately 20 g·L-1[3]. The yield and productivity of ABE fermentation process are low. Therefore, it is desirable to develop an effective butanol recovery method. A variety of recovery techniques, such as adsorption [4, 5], gas stripping [6, 7], membrane distillation, extraction and pervaporation (PV) [8-12] have been developed. Among these methods, pervaporation is considered to be particularly promising, because of its general advantages in environmental issue and energy-saving, particularly no harmful effects on microorganisms and no medium ingredients removed from the fermentation broth [13]. A lot of work has been done on recovering butanol from the ABE fermentation broth by pervaporation. One of the key points of this process is to prepare the pervaporation membranes with high permeability and selectivity. In our early work, polydimethylsiloxane (PDMS)/ceramic composite membrane is prepared by dip-coating the cross-linked PDMS layer on the tubular ceramic support. This type of membrane exhibited high performance, especially high permeate flux, for pervaporation of ethanol-water mixtures [14, 15] and removal of thiophene from model gasoline [16], compared to other PDMS membranes reported.
In this study, we adopt the PDMS/ceramic composite membrane for pervaporation of model butanol/ water mixtures in the concentration range in ABE fermentation process. The effects of operating temperature, feed concentration and feed flow rate on the membrane performance are investigated. The long- term stability of membrane is also examined.
2 EXPERIMENTAL
2.1 Materials
2.2 Preparation and characterization of PDMS/ ceramic composite membrane
The PDMS/ceramic composite membrane was prepared by conventional dip-coating method, which was similar to that in our previous work [14]. The PDMS polymer was dissolved in-heptane with a certain proportion, then the cross-linker TEOS and catalyst dibutyltin dilaurate were added into the polymer solution. The PDMS solutions were stirred at room temperature for 30 min and degassed under vacuum to be coating precursors. The ceramic support was sealed with two Teflon caps at both ends, then immersed slowly in the dip-coating solution for 60 s and pulled out of the solution at the same speed. Subsequently, the membrane was dried overnight at room temperature, and then cured at 120°C for 12 h to remove the residual solvent.
The surface and cross section morphologies of the PDMS/ceramic composite membranes were characterized by scanning electron microscope (SEM, QUANTA-2000). The dried composite membrane was fractured in liquid nitrogen and then sputtered with gold in vacuum. FT-IR spectra of the pristine PDMS and PDMS membrane were recorded in spectrophotometer (AVATAR-FT-IR-360, Thermo Nicolet, USA) with the range of 4000 to 400 cm-1, using the KBr disk technique. 32 scans were accumulated with a resolution of 2 cm-1for each spectrum.
2.3 Pervaporation experiment
Pervaporation experiment was conducted using a homemade apparatus [16]. The water bath was used to control the temperature of the feed tank. The permeate sample was collected in the cold trap. The butanol concentrations in the feed and the permeate side were analyzed using gas chromatography (GC-2014, SHIMADZU, Japan) equipped with a thermal conductivity detector, using a3 m×2 m stainless steel packed column and helium (He) as the carrier gas. In many cases, the butanol of permeate side separated into two phases. Under this circumstance, the permeate sample was diluted with deionized water to one phase prior to injection.
The permeate flux () at steady state is calculated using the following equation:
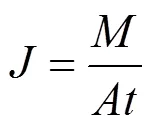
whereis the mass of the collected permeation, g·m-2·h-1;is the effective area of the membrane, m2; andis the collection time for the pervaporation, h.
The separation factor () of the PV membrane is defined as
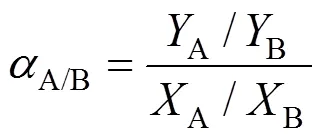
whereA,BandA,Bare the mass fractions of butanol and water in the permeate and feed side, respectively.
3 RESULTS AND DISCUSSION
3.1 Morphology and structure of PDMS/ceramic composite membrane
Figure 1 shows the SEM images of the surface (a) and cross section (b) of the PDMS/ceramic composite membrane. The membrane surface is dense and defect-free, and three-layer structures (from left to right, Al2O3layer, ZrO2layer, and PDMS layer, respectively) can be observed clearly from the cross section image (b). The PDMS layer with a thickness of about 10 μm is well adhered to the porous ceramic surface, with a part penetrated into its pores to form an interface layer, which will improve the interaction between PDMS layer and ceramic support [14].
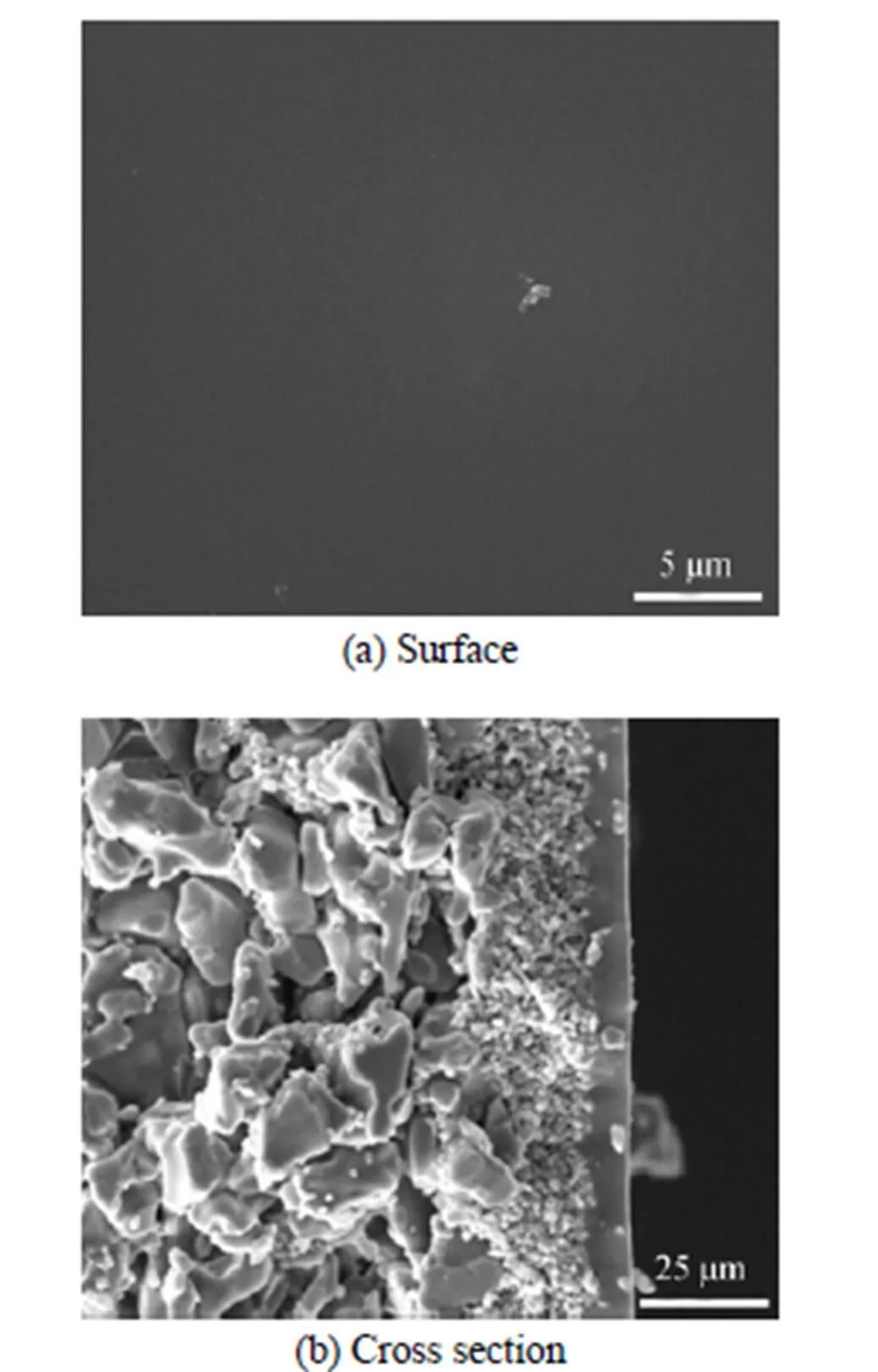
Figure 1 SEM images of the PDMS/ceramic composite membrane


Figure 2 FT-IR spectra of the pristine PDMS and PDMS membrane

3.2 Pervaporation performance of the PDMS/ ceramic composite membrane
3.2.1
The effect of operating temperature on PV performance of the PDMS/ceramic composite membrane is illustrated in Fig. 3. The total flux increases from 307 to 822 g·m-2·h-1as the temperature increases from 30 to 60°C. When the temperature increases, on the one hand, the rubbery PDMS swells and the polymer segments have more free volume and chain mobility; on the other hand, the vapor pressure difference is higher, which enhances the transport driving force. Both factors favor the diffusion of butanol and water molecules through the PDMS membrane, leading to higher permeate flux. However, since water molecule is smaller than that of butanol, the diffusion rate of water is faster, decreasing the separation factor slightly.
Generally, the temperature dependence of the total or partial flux (butanol or water flux) follows the Arrhenius expression [18]:
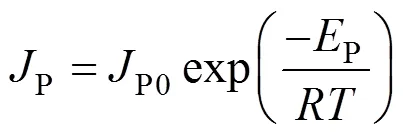
wherePis the total flux,P0is a constant,Pis the apparent activation energy for permeation,is the gas constant, andis the operating temperature in Kevin.
Figure 3 Effect of operating temperature on the PV performance of the PDMS/ceramic membrane (Feed mass concentration: 1% butanol)
■?separation factor;○?total flux
According to the slope of the Arrhenius plot presented in Fig. 4, the apparent activation energy values for water and butanol are obtained, which are 27.45 kJ·mol-1and 25.82 kJ·mol-1, respectively. It indicates that the water flux is more dependent on the temperature than that of butanol, so that the separation factor decreases as temperature increases.
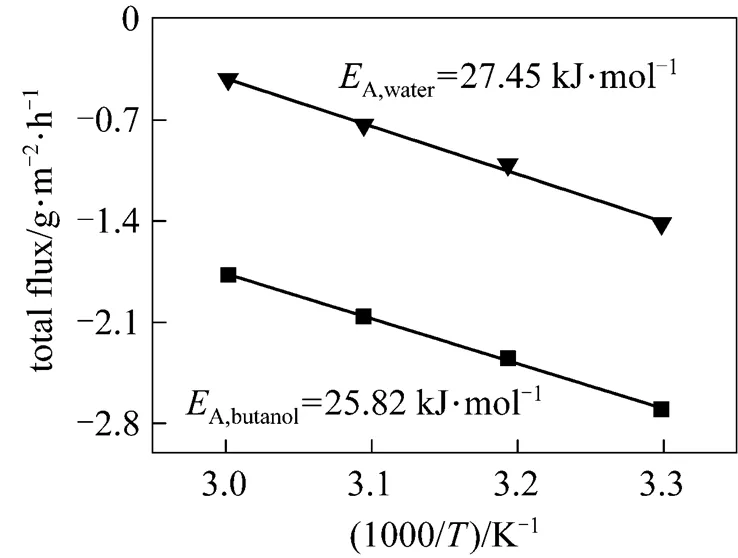
Figure 4 Arrhenius plots of partial flux for PDMS/ceramic membrane (Feed mass concentration: 1% butanol)
■?butanol;▼?water
3.2.2
The feed concentration is an important variable in the pervaporation. Fig. 5 shows the effect of the feed concentration of butanol on total flux and separation factor of the PDMS/ceramic composite membrane. As the butanol concentration increases, the total flux increases while the separation factor changes slightly. Increasing the butanol concentration in the feed facilitates the butanol sorption into the PDMS membrane and the PDMS chains swells more, which enhances the diffusion of the permeate components, increasing the total flux. Moreover, the diffusion rate of water is larger than that of butanol owing to the smaller molecule of water, decreasing the separation factor.
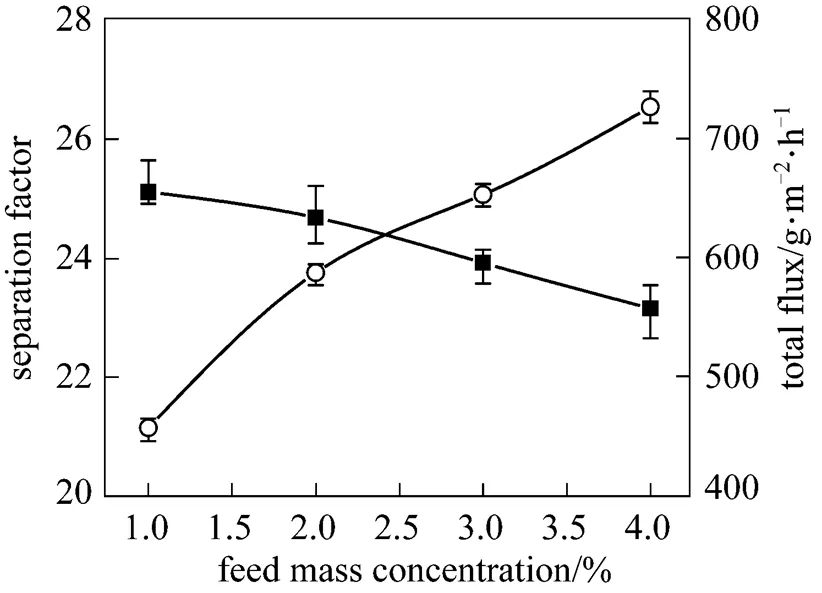
Figure 5 Effect of feed concentration on the PV performance of the PDMS/ceramic membrane (Temperature: 40°C)
■?separation factor;○?total flux
3.2.3
Figure 6 shows the effect of feed flow rate on the PV performance of the PDMS/ceramic composite membrane. The total flux increases with the flow rate, but the separation factor changes little. The increased feed flow rate reduces the thickness of the liquid boundary layer and the mass transport resistance in the pervaporation process, so that the total flux is increased. Due to the low butanol concentration in the feed, the concentration polarization is not obvious, so the flow rate has little effect on the separation factor.
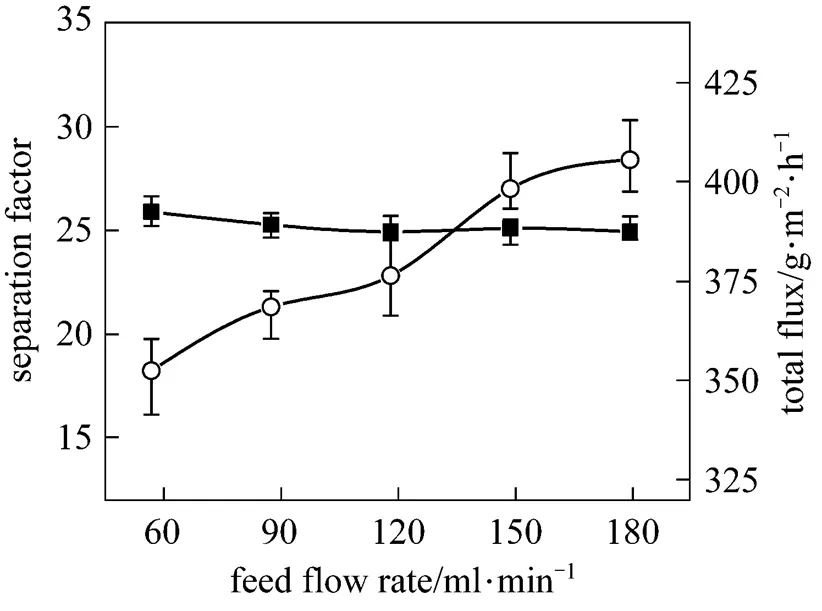
Figure 6 Effect of feed flow rate on the PV performance of the PDMS/ceramic membrane (Temperature: 40°C, feed mass concentration: 1% butanol)
■?separation factor;○?total flux
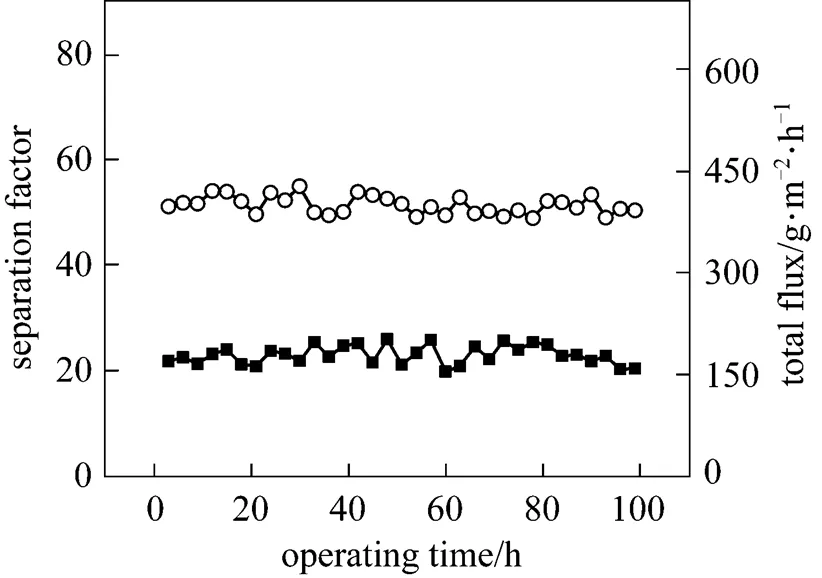
Figure 7 Effect of operation time on the PV performance of the PDMS/ceramic membrane (Temperature: 40°C, feed mass concentration: 1% butanol)
■?separation factor;○?total flux
3.2.4
The effect of the operating time on the PV performance of the PDMS/ceramic composite membrane was also studied. Fig. 7 shows that the total flux and separation factor change little over a period of 100 h. Different from the symmetric or organic/organic composite membranes, the PDMS layer was dip-coated on the inorganic ceramic support, which exhibits sufficient chemical, mechanical and thermal stability, forming a type of organic/inorganic composite membrane. Due to the ceramic supports and the interface layer between PDMS layer and ceramic support [Fig. 1 (b)], the three-dimensional swelling of the rubbery PDMS is restricted [19], which is beneficial to the long-term stability of the PDMS/ceramic composite membrane.

Table 1 Pervaporation performance of different membranes in butanol-water mixtures
3.3 Comparison of pervaporation performance with those in literature
Many researchers have investigated the recovery of butanol from its aqueous solution by pervaporation, and Table 1 shows the PV performance of different membranes in butanol-water mixtures. The PDMS/ceramic membrane in this work has a total flux of 457.4 g·m-2·h-1with the separation factor of 26.1 at 40°C for 1% (by mass) butanol in the feed. The total flux of the PDMS/ceramic composite membrane is much higher, while its separation factor is also relatively good. This is mainly attributed to the ceramic support with high porosity and the thin PDMS layer formed on the ceramic support, which reduces the transport resistance of the permeate components.
4 CONCLUSIONS
The prepared PDMS/ceramic composite membrane was applied for pervaporation removal of butanol from its dilute aqueous solution. The composite membrane shows high flux of 457.4 g·m-2·h-1and acceptable separation factor of 26.1 [1% (by mass) butanol in the feed at 40°C], and exhibits good long-term stability. Our study demonstrates that the PDMS/ceramic composite membrane will be useful for its application in the ABE fermentation-PV coupled process.
1 Schoutens, G.H., Groot, W.J., “Economic-feasibility of the production of-propanol-butanol-ethanol fuels from whey permeate”,., 20, 117-121 (1985).
2 Ezeji, T.C., Qureshi, N., Blaschek, H.P., “Acetone butanol ethanol (ABE) production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping”,..., 63, 653-658 (2004).
3 Qureshi, N., Ezeji, T.C., “Butanol, ‘a(chǎn) superior biofuel’ production from agricultural residues (renewable biomass): recent progress in technology”,.., 2, 319-330 (2008).
4 Yang, X.P., Tsao, G.T., “Enhanced acetone-butanol fermentation using repeated fed-batch operation coupled with cell recycle by membrane and simultaneous removal of inhibitory products by adsorption”,.., 47, 444-450 (1995).
5 Yang, X.P., Tsai, G.J., Tsao, G.T., “Enhancement of-adsorption on the acetone-butanol fermentation by-”,.., 4, 81-92 (1994).
6 Ezeji, T.C., Qureshi, N., Blaschek, H.P., “Production of acetone, butanol and ethanol byBA101 andrecovery by gas stripping”,..., 19, 595-603 (2003).
7 Ezeji, T.C., Karcher, P.M., Qureshi, N., Blaschek, H.P., “Improving performance of a gas stripping-based recovery system to remove butanol fromfermentation”,..., 27, 207-214 (2005).
8 Friedl, A., Qureshi, N., Maddox, I.S., “Continuous acetone-butanol-ethanol (ABE) fermentation using immobilized cells ofin a packed-bed reactor and integration with product removal by pervaporation”,.., 38, 518-527 (1991).
9 Favre, E., Nguyen, Q.T., Bruneau, S., “Extraction of 1-butanol from aqueous solutions by pervaporation”,...., 65, 221-228 (1996).
10 Groot, W.J., Vandenoever, C.E., Kossen, N., “Pervaporation for simultaneous product recovery in the butanol isopropanol batch fermentation”,.., 6, 709-714 (1984).
11 Boddeker, K.W., Bengtson, G., Pingel, H., “Pervaporation of isomeric butanols”,..., 54, 1-12 (1990).
12 Fadeev, A.G., Selinskaya, Y.A., Kelley, S.S., Meagher, M.M., Litvinova, E.G., Khotimsky, V.S., Volkov, V.V., “Extraction of butanol from aqueous solutions by pervaporation through poly(1-trimethylsilyl- 1-propyne)”,..., 186, 205-217 (2001).
13 Huang, J., Meagher, M.M., “Pervaporative recovery of-butanol from aqueous solutions and ABE fermentation broth using thin-film silicalite-filled silicone composite membranes”,..., 192, 231-242 (2001).
14 Xiangli, F.J., Chen, Y.W., Jin, W.Q., Xu, N.P., “Polydimethylsiloxane (PDMS)/ceramic composite membrane with high flux for pervaporation of ethanol-water mixtures”,...., 46, 2224-2230 (2007).
15 Xiangli, F.J., Wei, W., Chen, Y.W., Jin, W.Q., Xu, N.P., “Optimization of preparation conditions for polydimethylsiloxane (PDMS)/ ceramic composite pervaporation membranes using response surface methodology”,..., 311, 23-33 (2008).
16 Xu, R., Liu, G.P., Dong, X.L., Jin, W.Q., “Pervaporation separation of-octane/thiophene mixtures using polydimethylsiloxane/ceramic composite membranes”,, 258, 106-111 (2010).
17 Hijon, N., Manzano, M., Salinas, A.J., Vallet-Regi, M., “CaO-SiO2-PDMS coatings on Ti6Al4V substrates”,.., 17, 1591-1596 (2005).
18 Feng, X.S., Huang, R.Y.M., “Estimation of activation energy for permeation in pervaporation processes”,..., 118, 127-131 (1996).
19 Chen, Y.W., Xiangli, F.J., Jin, W.Q., Xu, N.P., “Organic-inorganic composite pervaporation membranes prepared by self-assembly of polyelectrolyte multilayers on macroporous ceramic supports”,..., 302, 78-86 (2007).
20 Jonquieres, A., Fane, A., “Filled and unfilled composite GFT PDMS membranes for the recovery of butanols from dilute aqueous solutions: influence of alcohol polarity”,..., 125, 245-255 (1997).
21 Vrana, D.L., Meagher, M.M., Hutkins, R.W., Duffield, B., “Pervaporation of model acetone-butanol-ethanol fermentation product solutions using polytetrafluoroethylene membranes”,..., 28, 2167-2178 (1993).
22 Liu, F.F., Liu, L., Feng, X.S., “Separation of acetone-butanol-ethanol (ABE) from dilute aqueous solutions by pervaporation”,..., 42, 273-282 (2005).
23 Jitesh, K., Pangarkar, V.G., Niranjan, K., “Pervaporative stripping of acetone, butanol and ethanol to improve ABE fermentation”,, 9, 145-154 (2000).
** To whom correspondence should be addressed. E-mail: wqjin@njut.edu.cn
2010-05-25,
2010-11-07.
the National Basic Research Program of China (2009CB623406), the National Natural Science Foundation of China (20990222), the Natural Science Foundation of Jiangsu Province (SBK200930313) and the “Six Kinds of Important Talents” Program of Jiangsu Province (2007007).
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-,?at 25 °C*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
- Alkylation of p-Cresol with tert-Butanol Catalyzed by NovelMultiple-SO3H Functioned Ionic Liquid*
