長形肉豆蔻Myristica argenteaWarb.化學(xué)成分研究
史 輯,趙啟苗,賈天柱
遼寧中醫(yī)藥大學(xué)藥學(xué)院,大連 116600
Introduction
Nutmeg is embodied by Chinese Phar macopoeia 2005. Now the market has two varieties of nu tmeg,one isM yristica fragransHoutt.,the other isM yristica argentea Warb.In Indonesia,M ystica fragransHoutt.is used for spices andM.argenteais used for medicine.While in China,it is different[1].So we carried out comprehensive study of chemical composition ofM.argentea.Previous phytochemical studies onM.argentearevealed the presence of essential oil and so on[2].In this paper,12 compounds were isolated and identified as safrole(1),methyleugenol(2),methylisoeugenol(3),3′-hydroxyisosafrole(4),7-hydroxy-3′,4′-methylene-flavan(5),terephthalic acid dimethyl ester(6),erythro-1,4-di(4-hydroxy-3-methoxyphenyl)-2,3-dimethylbutane(meso-dihydroguaiaretic acid(7),erythro-1-(4-hydroxy-3-methoxyphenyl)-4-(3, 4-methylenedioxy phenyl)-2,3-d imethyl butane(8),erythro-1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxy-4-(1(E)-propenyl)phenoxy)-propan-1-ol(9),nectandrin B(10), β-sitosterol(11),and daucosterol(12).Among of them,the compounds 4-6 and 8-12 were obtained from this plant for the first t ime.Compounds 4-6 were isola-ted firstly from this genus.
Exper imental
General
Melting point was determined on X-4 micromelting point apparatus and uncorrected.NMR spectra were recorded on a Bruker AV 300,Bruker DRX 600(1H: 600 MHz,13C:125 MHz)instrument with T MS as internal standard.MS spectra was taken on a HP MS 5973.Sephadex LH-20 (Pharmacia),and silica gel (200-300 mesh,Qingdao Ocean Chemical Co.Ltd. China)were used for column chromatographic separation.TLC was detected with 10%H2SO4-EtOH solution.
PlantMateria
The whole plant ofM ystica argenteawas purchased from Anhui Province of China and identified by Professor Zhai Yanjun,Institute of Identification of Traditional Chinese Medicine Development,Liaoning University of Traditional ChineseMedicine.The voucher spec imen was deposited in the KeyLaboratory of Traditional ChineseMedicine Processing Technology and Principle.
Extraction and Isolation
Dried and powdered whole plant ofM ystica argentea(8 kg)was extracted with 95% EtOH under reflux for three times.After removing solvent,the extractwas suspended in water and then extracted with petroleum ether(bp.60-90℃,10 L ×3 times),ethyl acetate (10 L ×3 times)and n-butanol(10 L ×3 times) respectively.The petroleum ether extract(250 g)was subjected by silica gel column chromatographywith petroleum ether-acetone(100∶1-1∶1)to give 20 fractions P1-P20.P5,P6,P10 was subjected by prepared TLC with hexane-acetone to give compound 1(50 mg),2 (100 mg)and 3(100 mg).The ethyl acetate extract (68 g)was subjected by silica gel column chromatographywith CHCl3-MeOH(100∶1-1∶1)to give 10 fractions E1-E10.Compound 1(50 mg)was obtained from fraction E-1 by crystallization with petroleum ether-EtOAc.Compound 11(100 mg)was obtained from E-1 by crystallization with MeOH.Fraction E-3 was chromatographed successively on silica gel eluted with CHCl3-Me2CO to give compounds 2(20 mg)and 3(40 mg).Fraction E-4 was separated with silica gel column eluting by CHCl3-MeOH(30∶1)to afford compound 4 (20 mg)and 5(10 mg).Using MeOH-H2O as eluents,fraction E-5 was separated by prepared HPLC to give compound 6(10 mg),7(8 mg),8(8 mg).Fraction E-6 and E-7 was separated by prepared HPLC with MeOH-H2O as eluents to give compound 9(10 mg) and 10(5 mg).Fraction E-9 was separated by silica gel column chromatography eluted with CHCl3-MeOH (10∶1)to give compound 12(100 mg).
Identification
Safrole(1) Yellow oil(petroleum ether).1H NMR (CDCl3,300 MHz)δ:6.72(1H,d,J=7.8 Hz,H-2), 6.01(1H,d,J=7.8 Hz,H-3),6.65(1H,s,H-6), 3.28(2H,d,J=6.6 Hz,H-7),5.94(1H,m,H-8), 5.02(1H,d,J=7.2 Hz,H-9a),5.06(1H,d,J=7.2 Hz,H-9b),5.94(2H,s,H-10).1H NMR data were identicalwith those of safrole[3]reported.
M ethyl eugenol(2) Pale yellow oil(petroleum ether).1H NMR(CDCl3,300 MHz)δ:6.78(1H,s,H-1),6.78(1H,s,H-2),6.74(1H,d,H-3),6.71(1H, s,H-6),3.3(2H,d,J=6.7 Hz,H-7),5.9(1H,m,H-8),5.0(2H,m,H-9),3.85(3H,s,H-10),3.86(3H, s,H-11).13C NMR(CDCl3,75 MHz)δ:137.6(C-1),132.5(C-2),120.3(C-3),147.2(C-4),148.8 (C-5),115.5(C-6),39.7(C-7),111.7(C-8),111.1 (C-9),55.8(C-10),55.6(C-11).The NMR data were in accordance with those of the literature[4].
M ethyl isoeugenol(3) Pale yellow oil(petroleum ether).1H NMR(CDCl3,300 MHz)δ:6.86(1H,d,J =7.0 Hz,H-2),6.76(1H,d,J=7.0 Hz,H-3),6.82 (1H,s,H-6),6.31(1H,d,J=15.0 Hz,H-7),6.08 (1H,m,H-8),1.84(3H,d,J=6.6 Hz,H-9),3.83 (3H,s,H-10),3.86(3H,s,H-11).The1H NMR data were in accordance with those of the reported[5].
3′-Hydroxyisosafrole(4) White crystal(petroleum ether-acetone),mp.62.5-63 ℃.EI-MS:m/z178 [M]+,with 135(100).1H NMR(CDCl3,600MHz)δ: 6.77(1H,d,J=7.8 Hz,H-2),6.83(1H,dd,J=7.8, 1.8 Hz,H-5),6.95(1H,d,J=1.8 Hz,H-6),6.54 (1H,d,J=15.6 Hz,H-1′),6.20(1H,dq,J=15.6 Hz,H-2′),4.31(2H,t,J=5.4,10.8 Hz,H-3′),5.97 (2H,s);13C NMR(CDCl3,125 MHz)δ:131.1(C-1), 126.7(C-2),148.0(C-3),147.3(C-4),131.0(C-5),121.2(C-6),108.3(C-7),105.7(C-8),63.8(C-9),101.1(C-10).The data ofMS and NMR were in accordance with those of reported[6].
(R)-form-7-hydroxy-3′,4′-methylene-flavan(5) White needles(petroleum ether),mp.120-124℃.EIMS:m/z270[M]+,148(100).[α]D– 14.2 (CHCl3).1H NMR(CDCl3,600 MHz)δ:4.95(1H, dd,J=10.2,2.4 Hz,H-2),2.15(1H,m,J=3.0,2.4 Hz,H-3a),2.05(1H,m,J=6.0,5.4,2.4,1.8 Hz, H-3b),2.90(1H,m,J=6.0,5.4,4.8 Hz,H-4a), 2.74(1H,m,J=3.6,3.0,1.8 Hz,H-4b),6.94(1H, d,J=7.8 Hz,H-5),6.88(1H,dd,J=7.8,1.8 Hz, H-6),6.41(1H,d,J=2.4 Hz,H-8),6.82(1H,d,J =7.8 Hz,H-2′),6.93(1H,d,J=1.2 Hz,H-3′), 6.40(1H,s,H-6′),5.98(2H,s,-CH2O-);13C NMR (CDCl3,125 MHz)δ:77.8(C-2),30.0(C-3),24.5 (C-4),130.2(C-5),106.7(C-6),157.4(C-7), 103.5(C-8),154.8(C-9),114.1(C-10),135.5(C-1′),107.9(C-2′),147.8(C-3′),147.2(C-4′), 108.2(C-5′),119.6(C-6′),101.1(CH2O).The data ofMS,[α]D,NMR were identical to those of literature[7].
Terephthalic acid d imethyl ester(6) White crystal (petroleum),mp.49-50℃ EI-MS:m/z194[M]+.1H NMR(CDCl3)δ:7.37(2H,q,J=3.6,3.0,2.4 Hz),7.55(2H,q,J=3.6,3.0,2.4 Hz),3.92(6H, s);13C NMR(CDCl3)δ:131.9(C-Ar),131.1(CHAr),128.7(CH-Ar),168.1(C=O),52.7(-OCH3).The data ofNMR andMSwere equal to those reported[8].
Erythro-1,4-di(4-hydroxy-3-methoxyphenyl)-2,3-d imethylbutane(m eso-dihydrogua iaretic acid(7) Colorless crystals(acetone),mp.84.5-85℃;EIMS:m/z330[M]+,we determined the molecular formula was C20H26O4according to itsMS and NMR data.1H NMR(CDCl3,600 MHz)δ:6.62(2H,d,J= 1.7 Hz,H-2,2′),6.83(2H,d,J=8.1 Hz,H-5,5′), 6.66(2H,dd,J=8.1,1.7 Hz,H-6,6′),2.30(2H, dd,J=13.5,9.3 Hz,H-7a,7′a),2.73(2H,dd,J= 13.5,5.1 Hz,H-7b,7′b),1.76(2H,m,H-8,8′), 0.85(2H,d,J=6.6 Hz,H-9,9′),3.86(6H,s), 5.46(2H,s,Ar-OH);13C NMR(CDCl3,125 MHz)δ: 133.8(C-1,1′),111.4(C-2,2′),146.3(C-3,3′), 143.5(C-4,4′),113.9(C-5,5′),121.7(C-6,6′), 39.2(C-7,7′),38.9(C-8,8′),16.2(C-9,9′),55.8 (-OCH3).The compound waserythro-1,4-di(4-hydroxy-3-methoxyphenyl)-2,3-dimethylbutane by comparison of the spectra properties with those reported previously[9-10].
Erythro-1-(4-hydroxy-3-methoxyphenyl)-4-(3,4-methylenedioxyphenyl)-2,3-d imethyl butane(8) Colorless needles(hexane-acetone),mp.68.5-69℃.EI-MS:m/z328[M]+,with a 137 as base peak.We deter mined the molecular formula was C20H26O4according to its MS and NMR data.C20H24O4,1H NMR (CDCl3,600 MHz)δ:6.67(1H,d,J=1.7 Hz,H-2), 6.84(1H,d,J=8.4 Hz,H-5),6.65(1H,dd,J=8.4, 1.8 Hz,H-6),2.73(1H,dd,J=13.6,9.3 Hz,H-7a),2.28(1H,dd,J=13.6,5.1 Hz,H-7b),1.77-1.72(1H,m,H-8),0.85(3H,d,J=5.1 Hz,H-9), 6.63(1H,d,J=1.7 Hz,H-2′),6.67(1H,d,J=7.8 Hz,H-5′),6.62(1H,dd,J=7.8,1.7 Hz,H-6′), 2.73(1H,dd,J=13.6,9.3 Hz,H-7′a),2.28(1H, dd,J=13.6,6.8 Hz,H-7′a),1.77-1.72(1H,m,H-8′),0.85(3H,d,J=6.8 Hz,H-9′),5.48(s,-OH), 5.93 (s,-OCH2O),3.88 (s,-OCH3);13C NMR (CDCl3,125 MHz)δ:133.7(C-1),109.4(C-2), 145.5(C-3),143.6(C-4),114.0(C-5),121.8(C-6),39.3(C-7),39.1(C-8),16.1(C-9),137.5(C-1′),107.9(C-2′),147.5(C-3′),146.4(C-4′), 111.4(C-5′),121.7(C-6′),39.4(C-7′),38.8(C-8′),16.2(C-9′),100.7(-OCH2O-),55.8(-OCH3) .The NMR andMS datawere identical to theerythro-1-(4-hydroxy-3-methoxyphenyl)-4-(3,4-methylenedioxyphenyl)-2,3-dimethyl butane reported[9-10].
Erythro-1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxy-4-(1(E)-propenyl)phenoxy)-propan-1-ol (9) Pale yellow oil.EI-MS:m/z344[M]+,164 (base peak).The1H NMR spectrums showed characteristic signals atδ1.89,ca.6.60 and 6.36(J=15.6, 6.6 Hz)due to a 1(E)-propenyl group.Further,the spectrum showed signals of one sec-methyl(δ1.20), two methoxyls(δ3.89 and 3.92,3H,respectively), twoα-andβ-methine protons(δ4.83,d,J=2.4 Hz;δ 4.34,dq,J=6.6,3.0 Hz)and five aromatic protons.The aromatic protons signal atδ6.99(1H,s), 6.92(1H,s)and the methoxyl signals atδ3.89(6H, s),aswell as the13C NMR signals indicated the presence of a 4-hydroxy-3-methoxyphenyl group in the molecule.In addition,the13C NMR spectrum indicated the presence of a 2-methoxy-4(1(E)-propenyl)-phenoxyl group.The smallJ7,8value 2.4 Hz and the chemical shift ofδC-7,C-8,and C-9 were 82.5,73.5 and 13.4 respectively,led to the structure oferythroshape,but notthreo.So these findings led to the structure of the compounderythro-1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxy-4-(1(E)-propenyl)phenoxy)-propan-1-ol .The data were1H NMR(CDCl3,600 MHz)δ:6.99 (1H,s,H-2),6.92(1H,s,H-5),6.90(1H,d,J=1.8 Hz,H-6),4.83(1H,d,J=2.4 Hz,H-7),4.34(1d, dq,J=6.6,2.4 Hz,H-8),1.20(3H,q,J=6.6,2.4 Hz,H-9),6.86(1H,d,J=1.2 Hz,H-3′),6.75(1H, dd,J=7.8,1.2 Hz,H-4′),6.97(1H,d,J=7.8 Hz, H-6′),6.37(1H,d,J=15.6 Hz,H-7′),6.17(1H, dd,J=15.6,6.6 Hz,H-8′),1.89(3H,d,J=6.6 Hz, H-8′),3.89(3H,s,-OCH3),3.92(3H,s,-OCH3), 5.59(-OH);13C NMR(CDCl3,125 MHz)δ:133.7 (C-1),119.0(C-2),146.4(C-3),145.6(C-4), 109.2(C-5),120.0(C-6),82.5(C-7),73.5(C-8), 13.4(C-9),144.7(C-1′),151.5(C-2′),113.9(C-3′),131.8(C-4′),108.8(C-5′),119.1(C-6′), 130.4(C-7′),125.0(C-8′),18.4(C-9′),55.8(-OCH3),55.9(-OCH3)[11-13].
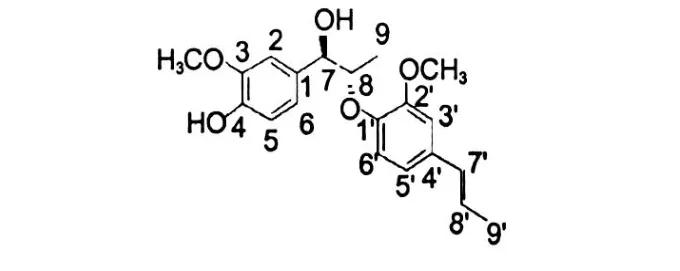
Fig.1 The structure of compound 9
Nectandrin B(10) Colorlessoil(acetone),EI-MS: m/z344[M]+(16),192(100),177(56),152 (18).1H NMR(CDCl3,600 MHz)δ:6.97(2H,d,H-2′,2′′),6.94(2H,dd,H-5′,5′′),6.90(2H,br d,H-6′,6′′),4.51(2H,d,J=6.0 Hz,H-2,5),2.34(2H, dq,J=6.6,4.8 Hz,H-3,4),1.05(2H,d,J=6.6 Hz,H-3-Me,4-Me),5.68(1H,brs,-OH),3.89(s,-OCH3);13C NMR(CDCl3,125 MHz)δ:134.1(C-1′, 1′′),109.1(C-2′,2′′),146.4(C-3′,3′′),145.0(C-4′,4′′),114.1(C-5′,5′′),119.2(C-6′,6′′),87.3 (C-2,5),44.2(C-3,4),12.9(C-3-Me,4-Me),55.8 (-OCH3).δH4.51(H-2,H-5)andJ=6.0 Hz indicated the compound was trans-,but not cis-(δH4.63,J= 9.2 Hz).So according to the exper imental formula,we deter mined the compound were nectandrin A,but not fragransin A2.

Fig.1 The structure of compound 10
β-Sitosterol(11) White needles(acetone),mp.
140-141℃.Reaction of Lieber man-Burchard(+).It was identified asβ-sitosterol,by comparision of mixed melting point andRfvalue with an authentic samples.
Daucosterol(12) White powder(methanol),mp. >300℃.Reaction of Lieber man-Burchard(+),reaction ofMolish(+).It was identified as daucosterol by comparision of mixed melting point andRfvalue with an authentic samples.
1 Chinese Pharmacopoeia Commission.Pharmacopoeia of the People′s Republic of China(中華人民共和國藥典),part I. Beijing:Chemical Industry Press,2005.1-1.
2 Chinese Material Medica editorial board.Chinese M aterial M edica(中華本草).Volume 7.Shanghai:Shanghai Science and Technology Press,1999.1599.
3 Aldrich Library of13C and1H FT NMR spectra.1992,2, 232B.
4 MiyazawaM,Kohno G.Suppression of chemicalmutagen-induced sos response by allylbenzen fromAsiasarum heterotropoidesin theSalm onella typh im urium ta1535/psk1002umu test.Nat Prod Res,2005,19(1):29-36.
5 Della Greca M,Monaco P,Previtera L,et al.Allelochemical activity of phenylpropanes fromAcorus gram ineus.Phytochem istry,1989,28:2319-2321.
6 Boberg EW,Miller EC,Miller JA.The metabolic sulfonation and side-chain oxidation of 3′-hydroxyisosafrole in the mouse and its inactivity as a hepatocarcinogen relative to 1′-hydroxysafrole.Chem B iol Interact,1986,59(1):73-97.
7 Ghosal S,Singh SK,Srivastava RS.Flavans fromZephyranthes flava.Phytochem istry,1985,24:151-153.
8 Aldrich Library of13C and1H FT NMR Spectra,1992,2, 1089A,1274B,127BA(NMR).
9 NakataniN,Ikeda K,KikuzakiH,et al.Diaryld imethylbutane lignans fromM yristica argenteaand their antimicrobial action againstStreptococcus mutans.Phytochem istry,1988,27: 3127-3129.
10 Kwon HS,K im MJ,Keong HJ,et al.Low-density lipoprotein (LDL)-antioxidant lignans fromM yristica fragransseeds. B ioorg M ed Chem Lett,2008,18:194-198.
11 Hada S,HattoriM,Tezuka Y,et al.New neolignans and lignans from the aril ofM yristica fragrans.Phytochem istry, 1988,27:563-568.
12 HattoriM,Hada S,Shu YZ,et al.New acyclic bis-phenylpropanoids from the aril ofM ystistica fragrans.Chem Phar m Bull,1987,35:668-674.
13 Kasahara H,MiyazawaM,Kameoka H.Absolute configuration of 8-O-4′-neolignans fromM yristica fragrans.Phytochem istry,1995,40:1515-1517.
14 HattoriM,Hada S,KawataY,et al.New 2,5-Bis-aryl-3,4-dimethyl tetrahydrofuran Lignan from the aril ofM yristica fragrans.Chem Phar m Bull,1987,35:3315-3322.

