Influence of A-type Zeolite on Methane Hydrate Formation*
ZANG Xiaoya (臧小亞), DU Jianwei (杜建偉), LIANG Deqing (梁德青),**, FAN Shuanshi (樊栓獅) and TANG Cuiping (唐翠萍)
?
Influence of A-type Zeolite on Methane Hydrate Formation*
ZANG Xiaoya (臧小亞)1,2,3, DU Jianwei (杜建偉)1,2,3, LIANG Deqing (梁德青)1,2,3,**, FAN Shuanshi (樊栓獅)2and TANG Cuiping (唐翠萍)1,2,3
1Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China2Guangzhou Center for Gas Hydrate Research, Chinese Academy of Sciences, Guangzhou 510640, China3Key Laboratory of Renewable Energy and Gas Hydrate, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China
The porous medium has an important effect on hydrate formation. In this paper, the formation process and the gas storage capacity of the methane hydrate were investigated with A-type zeolite and Sodium Dodecyl Sulfate (SDS) existing in the system. The results show that A-type zeolite can influence methane hydrate formation. At the temperature of 273.5 K and pressure of 8.3 MPa, the distilled water with A-type zeolite can form methane hydrate with gaseous methane in 12 hours. The formation process of the system with A-type zeolite was quite steady and the amount of A-type zeolite can influence the gas storage capacity significantly. The adding of A-type zeolite with 0.067 g·(g water)-1into 2×10-3g·g-1SDS-water solution can increase the gas storage capacity, and the maximum increase rate was 31%. Simultaneously the promotion effect on hydrate formation of 3A-type zeolite is much more obvious than that of 5A-type zeolite when the water adding amounts are 0.033 g·g-1and 0.067 g·g-1at the experimental conditions.
hydrate, formation, gas storage, A-type zeolite
1 INTRODUCTION
Gas hydrates (or clathrate hydrates) are ice-like nonstoichiometric compounds formed when light gases (such as methane, ethane,.) react with water at high pressures and/or low temperatures [1]. There are three main crystal structures I (SI), II (SII), and H (SH), which differ from each other in the cavity size and shape. At present, new technologies based on hydrates achieved significant developments not only in energy and environmental fields, but also in other practical topics [2-4], such as separation technologies. Because of the high gas storage concentration in theory, hydrates can also be used propitiously for carbon dioxide sequestration and storage or transportation of natural gas [5, 6]. Moreover, dissociation heat of clathrate hydrates is relative high, so it can be utilized for refrigeration applications in cold storage and air conditioning [7, 8].
However, the utilization of hydrates in these domains are restricted because of some disadvantages, such as low gas storage density, low formation rate and long induction time of gas hydrate formation. To solve these problems, extensive works were carried out to promote hydrate formation, such as enhancing the hydrate formation rate through agitation, using the ultrasonic wave to make water atomization, increasing the atmospheric-water contacted area, and so on [9, 10]. Several researchers focused on adding suitable surfactant to promote hydrate formation recently. Zhong and Rogers [11] noticed that sodium dodecyl sulfate (SDS) can accelerate the formation rate of natural gas hydrate. Zhang. [12] found that various kinds of additives such as alkylpolyglucside and potassium oxalate monohydrate increased the formation rates of natural gas hydrate(NGH) and its storage capacity, and reduced the induction time of NGH formation. Karaaslan. [13] studied the effect of anionic surfactant sodium-alkyl benzene sulfonate on the formation rate of methane hydrate, and methane and propane mixture hydrate. The results indicted that the production rate increases along with increasing surfactant. Gnanendran and Amin [14] also found that some proper supplements are helpful in increasing the gas storage capacity.
Porous medium have significant influence on hydrate formation rate, as they can reduce the chemical barrier which hydrate formation must overcome and promote hydrate formation [15, 16]. The A-type zeolite is a kind of porous medium. It has very widespread application in industry because of its special pores and the intensive polar adsorption function [17, 18]. Its tiny powder can provide large contact surfaces for hydrate nucleation. The effect of 3A-type zeolite on formation process of tetrahydrofuran(THF) hydrate has already been investigated [19], but the influence on methane hydrate formation and gas storage capacity has not been studied. Therefore, both the A-type zeolite and SDS were used as additives in this paper to investigate the effect of them on methane hydrate formation process and gas storage capacity.
2 EXPERIMENTAL
2.1 Experimental apparatus and materials
The experiments were carried out in the batch reactor made of stainless steel with a total volume of 100 ml. Fig. 1 shows the sketch of the experimental apparatus used for methane hydrate formation. The pressure transducer was bought from Guangzhou Senex Instrument Ltd., the type is DG-1215 and the precision is 0.1MPa. The thermocouple was bought from Guangzhou Deligen Instrument Company Ltd., the type is WZPK-193 and the precision is ±0.1 K.

Figure 1 Schematic drawing of the experimental apparatus
1—gas cylinder; 2—pressure-regulating valve; 3—pressure gauge; 4—gas reservoir; 5—pressure gauge; 6—mass flow meter; 7—vacuum pump; 8—output system; 9—mechanical agitation unit; 10—pressure transducer; 11—thermocouple; 12—hydrate formation reactor; 13—magnetic stirring unit; 14—water bath
The materials used are listed in Table 1.
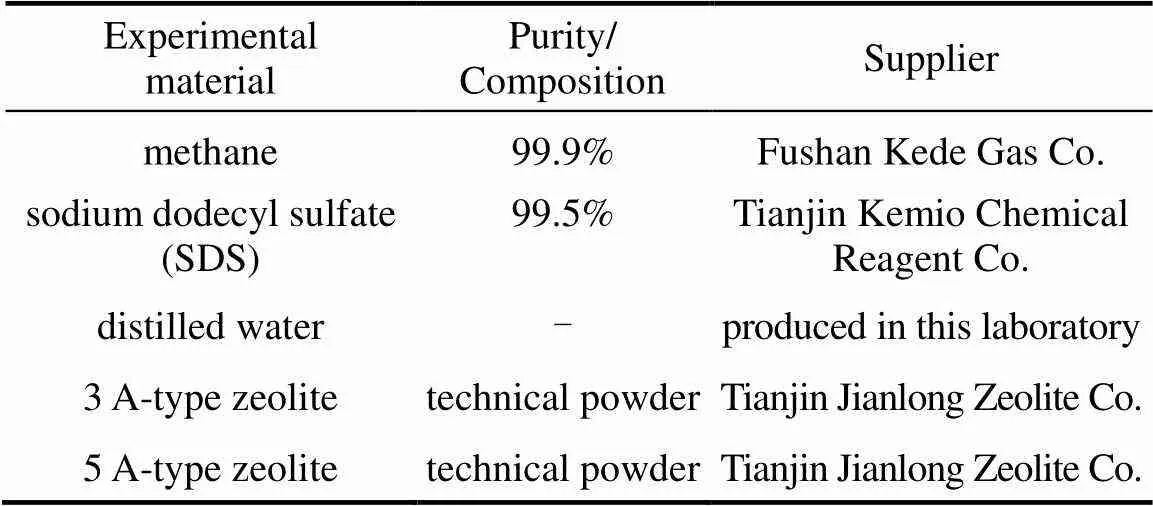
Table 1 List of experimental materials
2.2 Experimental procedure
Firstly, after the reactor cell was cleaned and dried, the SDS-water solution (or distilled water) and A-type zeolite were poured into the reactor cell with the mass concentration of the solution of 2×10-3g·g-1. After that, the cell was put into the water bath (the temperature was set at 273.5±0.5K) and connected with the gas pipeline. Then, the mechanical agitation (1000 r·min-1) and magnetic stirring (1300 r·min-1) were started up.
When the water bath temperature was stable, gaseous methane was charged into the cell after the pipeline was vacuumed and the pressure was 8.4 MPa. During the whole hydrate formation process, the temperature and pressure were recorded by the data collection system.
After the formation of methane hydrate was completed, the reactor cell was taken out from the water bath and the gaseous methane captured in hydrate was gathered by draining water to calculate the gas storage capacity.
3 RESULTS AND DISCUSSION
3.1 Influence of 3A-type zeolite on methane hydrate formation
The hydrate formation process is an exothermic reaction and usually experiences three stages: induction stage, rapid reaction stage and ending stage. If large amount of hydrate formed in a short time, the heat can not be transferred in time, and the temperature of the system will rise suddenly. But as shown in Fig. 2, the temperatures of the systems of 3A-type zeolite do not rise obviously except for the first three hours of formation process.
It is obviously shown in Fig. 2 that the temperatures raise when the high-pressure gaseous methane enters into the cool cell. The system temperatures rise after the gas intake process completed, which indicates that lots of methane hydrates form during this period. At the same time, the adding amount of 3A-type zeolite cannot influence significantly on temperature change in hydrate formation process. The temperatures with different quantities of 3A-type zeolite have similar change tendency and they overlap with each other. The gaseous methane can react completely with water to form methane hydrate over 12 hours under the experimental condition [20].

Figure 2 Reaction temperature of the systems with 3A-type zeolite

As shown in Fig. 2, the temperatures keep stable during the last 10 hours in formation process because of the special characteristic of 3A-type zeolite. 3A-type zeolite is one kind of crystal silicon aluminates with homogeneous aperture, polar adsorption function and extremely high specific surface area. The average aperture of 3A-type zeolite is 0.3 nm and water molecule can enter into interior. Therefore, the methane hydrate hydration process can occur separately on each tiny zeolite powder. 3A-type zeolite powders can provide the nucleus for the methane hydrate formation, reduce the nucleation randomicity and promote hydrate formation. Under the function of 3A-type zeolite, methane hydrate hydration process carries on slowly and the formation rate keeps constant basically. The reaction heat can be transferred in time, so the system temperature maintains stable.
3.2 Influence of 5A-type zeolite on methane hydrate formation
Figure 3 shows the temperature and pressure variation during methane hydrate formation process of 2 g 5A-type zeolite powder and 30ml distilled water system. The temperatures and pressures of the other systems with different amount of 5A-type zeolite are similar. It is evidently that the distilled water with the existence of 0.067 g·(g water)-15A-type zeolite powder is easy to react with gaseous methane under the experimental condition. Moreover, the reaction process is steady at preliminary formation stage, which is different with 3A-type zeolite system. After the reaction experiences 5 hours, system temperature has a small undulation and the duration is about 1 hour. This phenomenon indicates that the hydrate formation rate increases at this stage. Afterward, the temperature maintains constant until the reaction is finished. Because the reactor cell is connected with gas reservoir and its volume is much smaller than gas reservoir, the pressure in the reactor cell is equal to that in gas reservoir. The gaseous methane consumed in reaction is neglectable, so the pressure does not have much decrease during the whole reaction. It also can be seen from Fig. 3 that temperature does not vary obviously in the rest of the reaction process, which indicates that the hydration rate maintains constant.
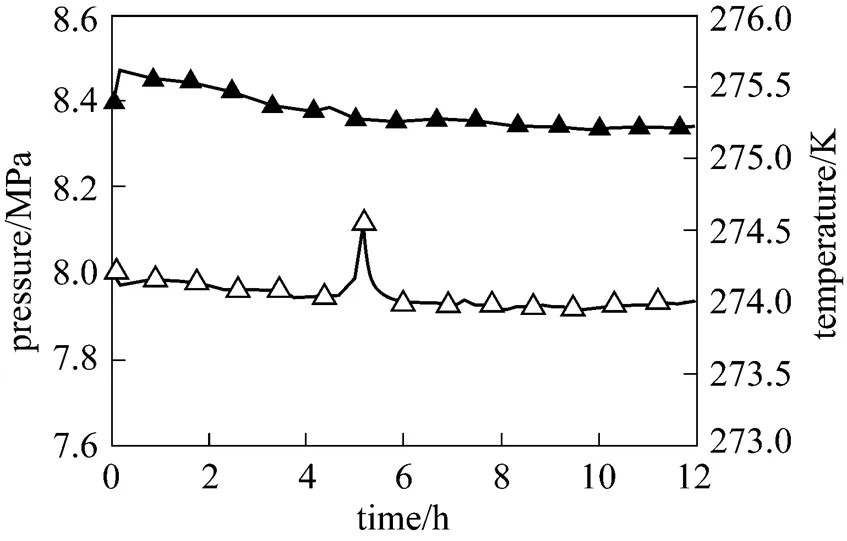
Figure 3 Reaction curve of the system with 5A-type zeolite
▲?pressure;△?temperature
Figure 4 is the temperature and pressure curves during methane hydrate formation process of 30 ml SDS-water solution system. As shown in Fig. 4, the SDS-water solution is easy to react with gaseous methane in the preliminary methane hydrate formation stage. The pressure starts to decrease rapidly in 5 min after the gas intake process is completed, indicating that a large amount of methane hydrates is formed at this stage. 1 h later, the decrease range of pressure lessens and the pressure maintains stable till the end. And the temperature also keeps constant till the end.

Figure 4 Reaction curve of the system with SDS-water solution
▼?pressure;▽?temperature
The temperature and pressure curves during methane hydrate formation of 2 g 5A-type zeolite and 30 ml SDS-water solution system are shown in Fig. 5. In preliminary stage, the interior temperature rises during the period of compressed gas entering and then drop rapidly. This process lasts about 40 min and is similar to Fig. 4. This is because both 5A-type zeolite and SDS can promote the methane hydrate formation rate in preliminary stage. Methane hydrate forms quickly and the reaction heat accumulates in cell, leading to the temperature rising about 20 min. After that, the effect of 5A-type zeolite becomes weaken. Hydrate formation rate tends to be steady and the reaction heat can be taken away by water bath in time, and thus the temperature maintains constant simultaneously.
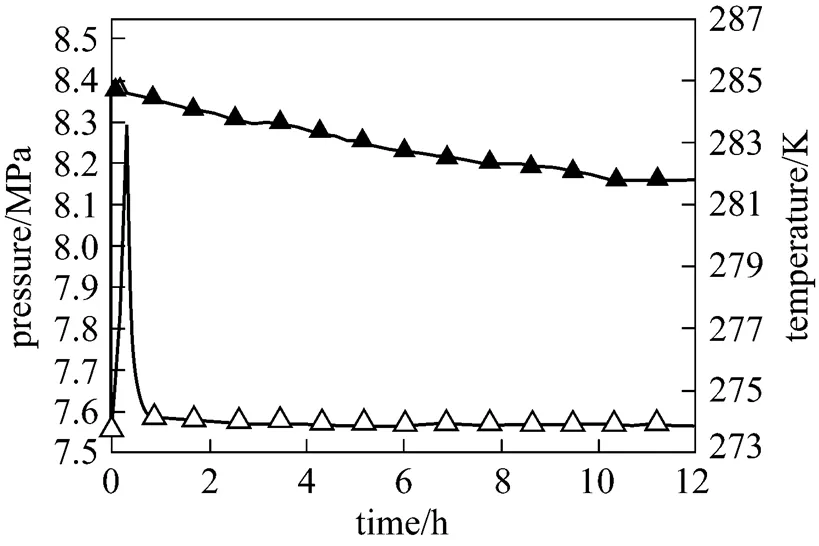
Figure 5 Reaction curve of the system with 5A-type zeolite and SDS-water solution
▲?pressure;△?temperature
3.3 Gas storage capacity variation
The gas storage capacity of methane hydrate at experimental temperature and pressure are summarized in Tables 2-5.
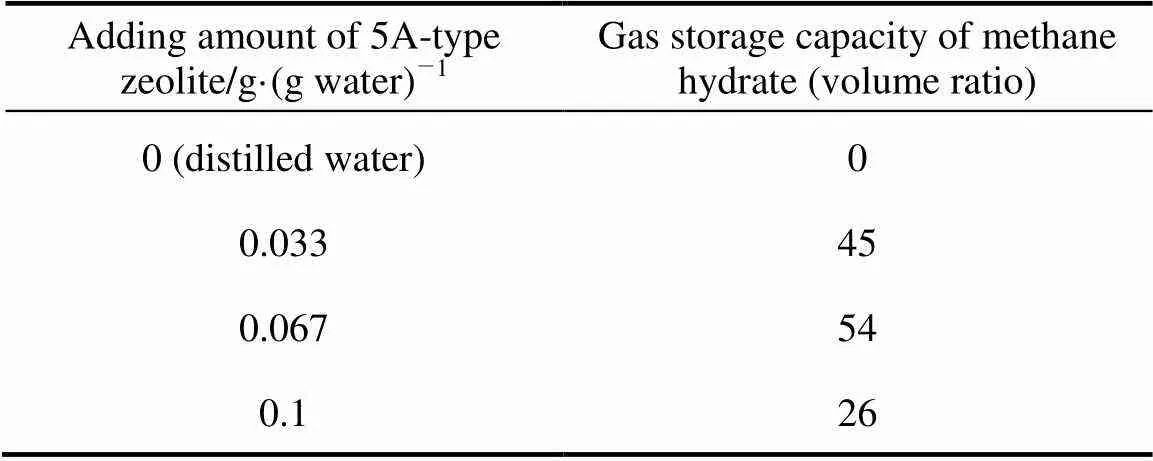
Table 2 Gas storage capacity of 5A-type zeolite and distilled water system

Table 3 Gas storage capacity of 3A-type zeolite and distilled water system
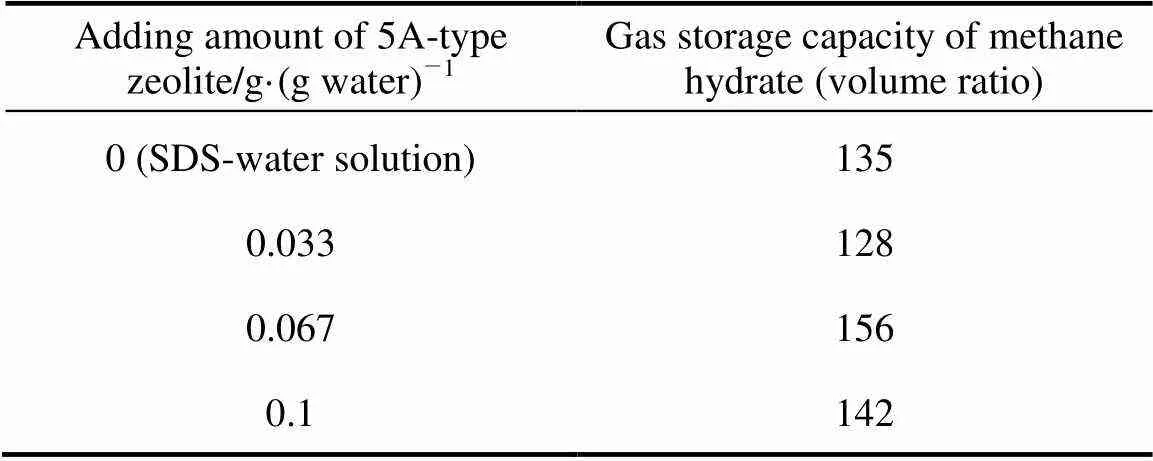
Table 4 Gas storage capacity of 5A-type zeolite and SDS-water system
As shown in Tables 2 and 3, the adding of 3A-type and 5A-type zeolite can promote methane hydrate formation and the amount of zeolite powder can change the gas storage capacity significantly. The gas storage capacity of methane hydrate is 49 (volume ratio) when the adding amount of 3A-type zeolite powder is 0.033g·(g water)-1. When the quantity increases to 0.067 g·(g water)-1, the gas storage capacity enhances to 54 (volume ratio) correspondingly. But it starts to decrease when the quantity of 3A-type zeolite increases to 0.1 g·(g water)-1. These results indicate that 0.067 g·(g water)-1of 3A-type zeolite powder can promote methane hydrate formation effectively. The gas storage capacity of methane hydrate reaches maximum when 0.067 g·(g water)-1of 3A-type zeolite powder is added in the system.
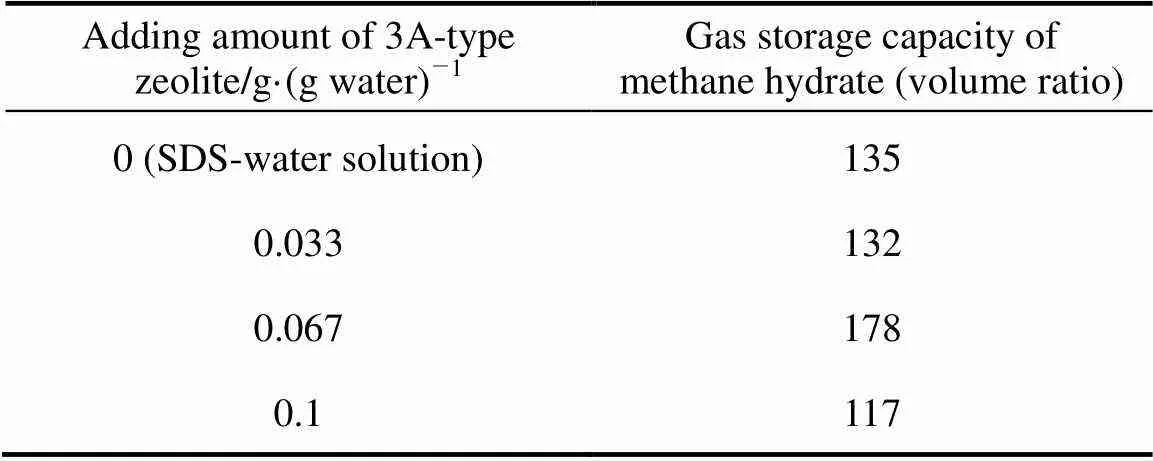
Table 5 Gas storage capacity of 3A-type zeolite and SDS-water system
Tables 4 and 5 show the gas storage capacity of methane hydrate of A-type zeolite and 30 ml SDS-water solution system. Similar with Tables 3 and 4, the methane hydrate has maximum gas storage capacity when the adding amount of A-type zeolite is 0.067 g·(g water)-1. Under the experimental condition, the gas storage capacity of hydrate formed with 2×10-3g·g-1SDS-water solution and methane gas is 135 (volume ratio), and it starts to decrease when the 3A-type zeolite powder adding is 0.033 g·(g water)-1or 0.1 g·(g water)-1. But the existence of 0.067 g·(g water)-1or 0.1 g·(g water)-15A-type zeolite can increase the gas storage capacity obviously and the maximum increase rate is about 31%.
These results indicate that the quantity of A-type zeolite powder has significant influence on gas storage capacity of methane hydrate in 2×10-3g·g-1SDS-water solution system, and there is an optimize adding amount of A-type zeolite which can maximize the gas storage capacity. The reasons are probably that the porous cage structure of A-type zeolite adsorbs much water and changes the SDS-water solution concentration. A-type zeolite loses activity and it cannot adsorb methane gas molecule anymore, and then the adsorption effect of A-type zeolite is greatly weakened. In addition, 3A-type zeolite is the Kalium A type zeolite and 5A-type zeolite is the Calcium A type zeolite. Both K+and Ca2+can dissolve into the water, form an electrolyte solution and change the electric and magnetic environment of the SDS-water solution. Both of the two reasons play an important role in promoting the gas storage capacity of methane hydrate when the A-type zeolite quantity is 0.067 g·(g water)-1, so that the gas storage capacity increases compared to SDS-water system. But the gas storage capacity will drop if the quantity is not suitable.
Generally speaking, the gas storage capacity of the methane hydrate formed in 3A-type zeolite system is higher than that formed in 5A-type zeolite system with the same condition.
3.4 Mechanism analysis about the influence of 3A and 5A type zeolites
Methane hydrate formation process is extremely stable with the existence of 3A-type zeolite, and the temperature in the cell has not change apparently in the formation process except for the preliminary stage. These results indicate that the methane hydrate formation rate keeps constant.
On the other hand, the adding of A-type zeolite can promote methane hydrate formation. It also can cause the distilled water to react with methane gas to form hydrate. Simultaneously optimum amount of A-type zeolite and SDS together can promote hydrate formation significantly, but improper quantity of A-type zeolite may play negative roles in hydrate formation process.
The reason can be analyzed from below aspects. The inner pore size of 3A and 5A type zeolites are 0.3 nm and 0.5 nm, and the molecule dynamic diameters of methane and water are approximately 0.436 nm and 0.29 nm, respectively. Both 3A and 5A type zeolites can adsorb water molecule, but only 5A type zeolite can adsorb methane molecule under the experimental condition. These are the difference between 3A and 5A type zeolites in promotion effect on methane hydrate formation. 3A type zeolite can adsorb the water molecule to provide nucleation grain, reduce the formation randomicity and change the electric and magnetic environment of the SDS-water solution. Its pores cage can supply third contact surface between water and methane molecular, reduce the surface energy and chemistry potential barrier which the formation must overcome. Thus, it has positive effect on the hydrate formation. In other words, 3A-type zeolite plays a role as the reaction intermedium in promoting hydrate formation. Simultaneously, 3A-type zeolite disperses in all of the solution and the solution is isotropic, and the formation rate mostly depends on the rate of gas solubility in water. The methane gas molecules dissolve in water and react with water slowly and orderly at the function of 3A-type zeolite. This is why there is no obvious temperature and pressure variation in hydrate formation process of 3A-type zeolite.
But the existence of 5A-type zeolite can change the solution characteristic and adsorb the methane gas molecule. These are the reasons why the temperature rises in gas intake preliminary stage in Fig. 5. At the gas intake preliminary stage, 5A-type zeolite adsorbs many methane molecule and SDS-water solution. It plays a key role on methane hydrate formation rate because of its dual polar adsorption effect, leading to an increase in the methane hydrate formation rate and the interior temperature. When large numbers of 5A-type zeolite pores are occupied by the methane molecule, the 5A-type zeolite starts to lose activity. Therefore, the promotion effect of 5A-type zeolite on methane hydrate formation becomes weak, and the methane hydrate formation rate slows down and tends to be stable.
In conclusion, the porous medium characteristic and the pore size are the crucial effects on hydrate formation. The polar adsorption ability of A-type zeolite also makes the formation easier because it can reduce the surface energy and chemistry potential barrier which the hydrate formation must overcome. Therefore, the gas storage capacity of 3A-type zeolite system is higher than that of 5A-type zeolite under the same condition in generally.
4 CONCLUSIONS
The results from this study show that the existence of A-type zeolite can promote methane hydrate formation and enable pure distilled water to react with gaseous methane to form hydrate when the temperature is 273.5 K and the pressure is 8.3 MPa. Methane hydrate formation process of the water with 3A-type zeolite is stable with both the temperature and pressure having no significant change.
The promotion effect of 3A-type zeolite on hydrate formation is much more significant than 5A-type zeolite when the water adding quantity is 0.033 g·g-1or 0.067 g·g-1. The amount of A-type zeolite can influence the gas storage capacity of methane hydrate obviously. The methane hydrate has maximum gas storage capacity when the adding amount of A-type zeolite powder is 0.067 g·(g water)-1in all of the experiments with the maximum increase of about 31%.
1 Sloan, E.D., Clathrate Hydrates of Natural Gases, 2nd ed., Marcel Dekker, New York (1997).
2 Fan, S.S., Storage and Transport Technology of Natural Gas Hydrate, Chemical Industry Press, Beijing (2005).
3 Chatti, I., Delahaye, A., Fournaison, L., Petitet, J.P., “Benefits and drawbacks of clathrate hydrates: A review of their areas of interest”,.., 46 (9/10), 1333-1343 (2005).
4 Luo, Y.T., Zhu, J.H., Chen, G.J., “Numerical simulation of separating gas mixtureshydrate formation in bubble column”,...., 15 (3), 345-352 (2007).
5 Yang, H.Q., Xu, Z.H., Fan, M.H., Gupta, R., Slimane, R.B., Bland, A.E., Wright, I., “Progress in carbon dioxide separation and capture: A review”,.., 20 (1), 14-27 (2008).
6 Loveday, J.S., Nelmes, R.J., “High-pressure gas hydrates”,...., 10, 937-950 (2008).
7 Rogers, R.E., Yevi, G., Swalm, M., “Hydrates for storage of natural gas”, In: Second International Conference on Natural Gas Hydrate, Toulouse, 423-429 (1996).
8 Isobe, F., Mori, Y.H., “Formation of gas hydrate or ice by direct-contact evaporation of CFC alternatives”,..., 15 (2), 137-142 (1992).
9 Ribeiro, C.P., Lage, P.L.C., “Modelling of hydrate formation kinetics: State-of-the-art and future directions”,..., 63 (8), 2007-2034 (2008).
10 Guo, T.M., Wu, B.H., Zhu, Y.H., Fan, S.S., Chen, G.J., “A review on the gas hydrate research in China”,...., 41, 11-20 (2004).
11 Zhong, Y., Rogers, R.E., “Surfactant effects on gas hydrate formation”,..., 55, 4175-4187 (2000).
12 Zhang, C.S., Fan, S.S., Liang, D.Q., Guo, K.H., “Effect of additives on formation of natural gas hydrate”,, 83, 2115-2121 (2004).
13 Karaaslan, U., Uluneye, E., Parlaktuna, M., “Effect of an anionic surfactant on different type of hydrate structures”,..., 35 (1), 49-57 (2002).
14 Gnanendran, N., Amin, R., “The effect of hydrotropes on gas hydrate formation”,..., 40 (1), 37-46 (2003).
15 Cha, S.B., Ouar, H., Wildeman, T.R., Sloan, E.D., “A third-surface effect on hydrate formation”,..., 92, 6492-6494 (1988).
16 Yan, L.J., Chen, G.J., Pang, W.X., Liu, J., “Experimental and modeling study on hydrate formation in wet activated carbon”,..., 109, 6025-6030 (2005).
17 Xu, R.R., Pang, W.Q., Chemistry-Zeolites and Porous Materials, Science Press, Beijing, 34 (2004). (in Chinese)
18 Tian, J., Zhang, M.H., Dong, X.Q., “Adsorption and diffusion equilibrium research about water on 3A molecular sieve”,... (), 33 (10), 932-936 (2004). (in Chinese)
19 Zang, X.Y., Fan, S.S., Liang, D.Q., Li, D.L., Chen, G.J., “Influence of 3A molecular sieve to tetrahydrofuran(THF) hydrate formation”,...., 51 (9), 893-900 (2008).
20 Sun, Z.G., Ma, R.S., Guo, K.H., Fan, S.S., Wang, R.Z., “Natural gas storage in hydrates with the presence of promoters”,.., 44 (17), 2733-2742 (2003).
2009-04-10,
2009-08-09.
the National Natural Science Foundation of China (50876107), the National Basic Research Program of China (2009CB219504), NSFC-Guangdong Union Foundation (NSFC-U0733033) and CAS Program (KGCX2-YW-805).
** To whom correspondence should be addressed. E-mail: liangdq@ms.giec.ac.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
