Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
CHANG Chun (常春)**, MA Xiaojian (馬曉建) and CEN Peilin (岑沛霖)
?
Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
CHANG Chun (常春)1,**, MA Xiaojian (馬曉建)1and CEN Peilin (岑沛霖)2
1School of Chemical Engineering, Zhengzhou University, Zhengzhou 450002, China2School of Material Science and Chemical Engineering, Zhejiang University, Hangzhou 310027, China
Levulinic acid is considered as a promising green platform chemical derived from biomass. The kinetics of levulinic acid accumulation in the hydrolysis process of wheat straw was investigated in the study. Using dilute sulfuric acid as a catalyst, the kinetic experiments were performed in a temperature range of 190-230°C and an acid concentration range of 1%-5% (by mass). A simple model of first-order series reactions was developed, which provided a satisfactory interpretation of the experimental results. The kinetics of main intermediates including sugar and 5-hydroxymethylfurfural (5-HMF) were also established. The kinetic parameters provided useful information for understanding the hydrolysis process.
levulinic acid, wheat straw, hydrolysis, kinetics
1 INTRODUCTION
Biomass has been identified as an important source for fuel alternatives and value-added chemicals, which has an annual production up to 2×1011tons in the world [1, 2]. Many researches are being carried out worldwide to convert biomass into new platform chemicals, among which levulinic acid (LA) has drawn much attention. LA is a short chain fatty acid having a ketone carbonyl group and an acidic carboxyl group, which can be used for the synthesis of various organic compounds. The ester of LA can either be used in the flavoring and fragrance industry or as blending component in biodiesel [3, 4]. Therefore, LA has the potential to be an important basic chemical material.
LA can be produced from biomass by acid hydrolysis. Under acidic condition at elevated temperatures, the biomass decomposes into a variety of products, with LA and formic acid being the final soluble products [5]. In the process of hydrolysis, the reaction temperature and acid concentration play important roles in the final LA yield. Especially, higher LA yield can be obtained at higher temperature and lower acid concentration. It was reported that the LA yield at 200°C was significantly higher than that at 160°C with whole kernel grain sorghum as raw materials [6], and similar results was obtained using corn starches as materials [7]. At higher temperature, dilute acid will accelerate the biomass decomposition. In Biofine process, a two-step hydrolysis with dilute mineral acid (1%-5%) is available, and the final LA yield is more than 70% of theoretical yield, one of the highest reported in the literature [8].
Although the effects of temperature and acid concentration on LA formation were investigated intensively [9-11], the mechanism and kinetics of LA formation are not understood clearly, and no model is available for the optimization. Some researchers tried to elucidate the mechanism of LA formation by using glucose as the representative of biomass [12], but obtained different kinetic data because of the complexity of biomass. Some papers were focused on kinetics and mechanism of LA formation from biomass at lower temperature [13], 80-98°C, but few were at higher temperature. Recently, Girisuta. [14, 15] carried out kinetic experiments with microcrystalline cellulose and water hyacinth plant separately in a temperature range of 150-200°C and developed a kinetic model. It is needed to study the kinetics of LA formation in higher temperature range.
Wheat straw is a kind of renewable, cheap and widely available biomass. The hydrolysis of wheat straw to produce LA is a good alternative for this abundant resource. The optimal condition for the LA production from wheat straw has been investigated in our previous work [16]. The objective of this work is to study the kinetics of LA formation in higher temperature range at lower acid concentrations with wheat straw as raw material.
2 Experimental
2.1 Material and methods
Wheat straw from a local farm (Zhengzhou, China) was used as raw material. The air-dried wheat straw was sieved and the portion under 0.161 cm2was collected for tests. The main composition of wheat straw samples was cellulose 40.35%, hemicellulose 25.57% and ligin 22.30%. Sulfuric acid was in analytical purity, and deionised water was used for all reactions.
The hydrolysis experiments were conducted in a 0.5 liter stainless steel (316 L) batch reactor with the nominal pressure of 10 MPa and temperature of 300°C. A thermocouple probe inside the reactor was connected to a digital temperature indicator, with the accuracy of ±0.5°C. Each run of tests started with wheat straw and water in the reactor heated to the desired temperature, and then the acid was injected into the reactor under the pressure of N2from the cylinder. The stirrer speed was adjusted to 600 r·min-1. The hydrolysis experiments were conducted at 190, 210 and 230°C. The concentration of sulfuric acid was at 1%, 3% and 5% (by mass), and the liquid to solid ratio was maintained at 16︰1. After certain reaction time, the reaction product was discharged into a cooler, and the liquid product was sampled and analyzed.
2.2 Analysis
The concentration of remaining monosaccharide, 5-hydroxymethylfurfural (5-HMF) and LA were analyzed. Monosaccharide in the hydrolysate was determined by the 3,5-dinitrosalicylic acid (DNS) method. 5-HMF was analyzed by a spectrophotometer method, and the concentration of LA was determined by gas chromatography with a flame ionization detector [17].
3 RESULTS AND DISCUSSION
3.1 Kinetic models
Under acidic condition at elevated temperatures, biomass decomposes into a variety of products, with LA and formic acid being the final products through the intermediates such as monosaccharide and 5-HMF [5]. Due to the complexity of the hydrolysis process, simplified model is usually used to determine the kinetics of biomass decomposition. Hence, we make some assumptions based on the related results [5, 15]. (1) The formation of intermediates is negligible, and the hydrolysis is a series of irreversible reactions from biomass to monosaccharide, then to 5-HMF, and finally to LA. (2) All the unknown products and humic solids are byproducts. (3) LA is mainly produced by decomposition of 5-HMF, and other possible reaction routines are negligible. Based on the assumptions, a pseudo-homogeneous irreversible first-order reaction model is proposed as shown in Fig. 1.
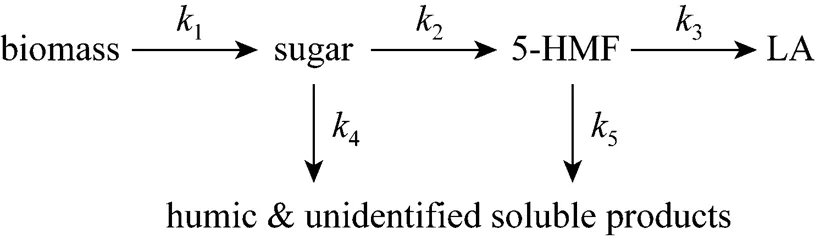
Figure 1 Simplified model of LA formation from biomass decomposition
Based on the model, the following set of differential equations can be obtained:
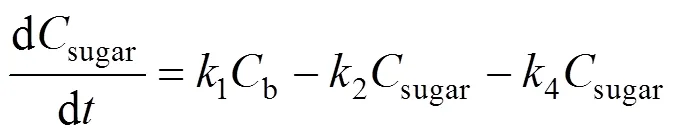
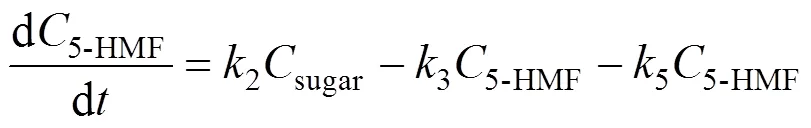
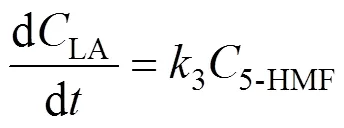
Solving the differential equations, we obtain an analytic expression of concentration of monosaccharide, 5-HMF and LA.
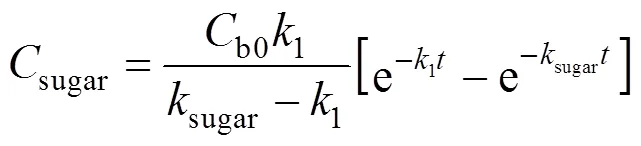
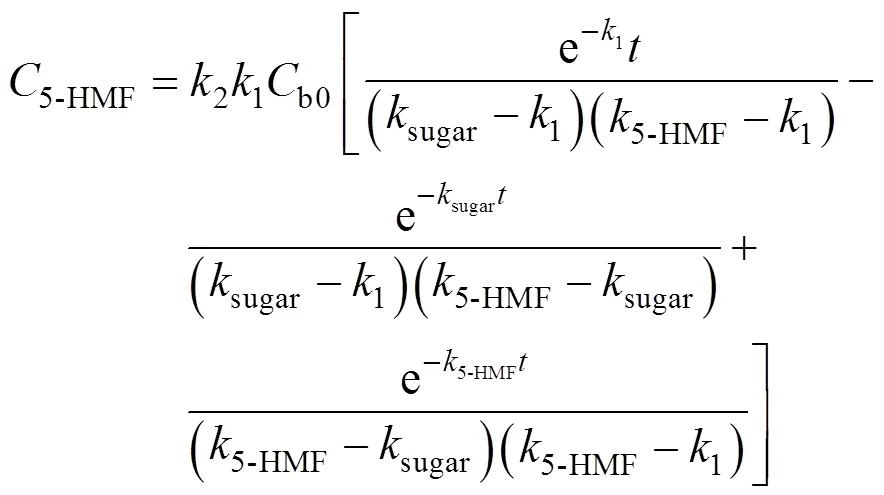
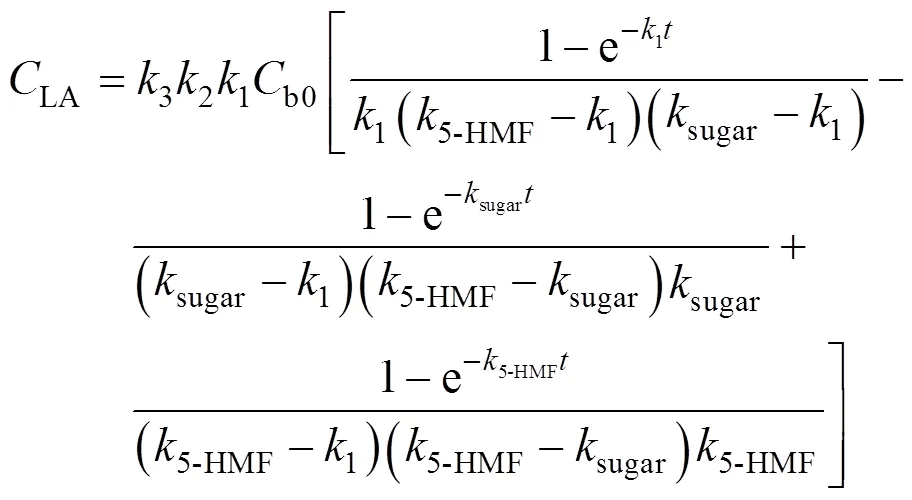
The kinetic coefficients can be correlated with temperature by applying modified Arrhenius equations for the effect of acid concentration [18]:
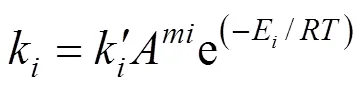
Equations (1)-(7) were used to fit the experimental data, and the parameters were evaluated using the method of non-linear least squares regression analyses by MATLAB 6.5.
3.2 Kinetic model of sugar
Sugar is a secondary product obtained in the hydrolysis of wheat straw. The kinetic model of sugar concentration is described by Eq. (4), which has the same form as Saeman’s model [19]. Table 1 shows the kinetic parameters for the hydrolysis of wheat straw and Fig. 2 shows the experimental and predicted data for this hydrolysis.
Figure 2 shows that the sugar concentration reaches a maximum value and then decreases with reaction time in all hydrolysis processes. Both the formation rate and decomposition rate of sugar increase quickly with temperature and acid concentration, which is also shown by the increase of reaction rate1andsugarin Table 1. The relative rate of sugar formation with respect to sugar decomposition is enhanced by increasing the temperature and acid concentration, as indicated by an increasing1/sugarratio from Table 1. Therefore, higher temperature and acid concentration will promote the hydrolysis of wheat straw. The values of2in Table 2 show a good agreement between predicted and experimental data for all regressions, so that Saeman’s model is corroborated. The average values of1andsugarare 78.66 and 61.06 kJ·mol-1, respectively. The kinetic models permit the prediction of the reaction time for the maximum values of sugar. At 5% H2SO4and 230°C, the maximum concentration of sugar (19.70 g·L-1) is obtained at 0.9 min.

Table 1 Kinetic parameters of sugar release for the hydrolysis of wheat straw
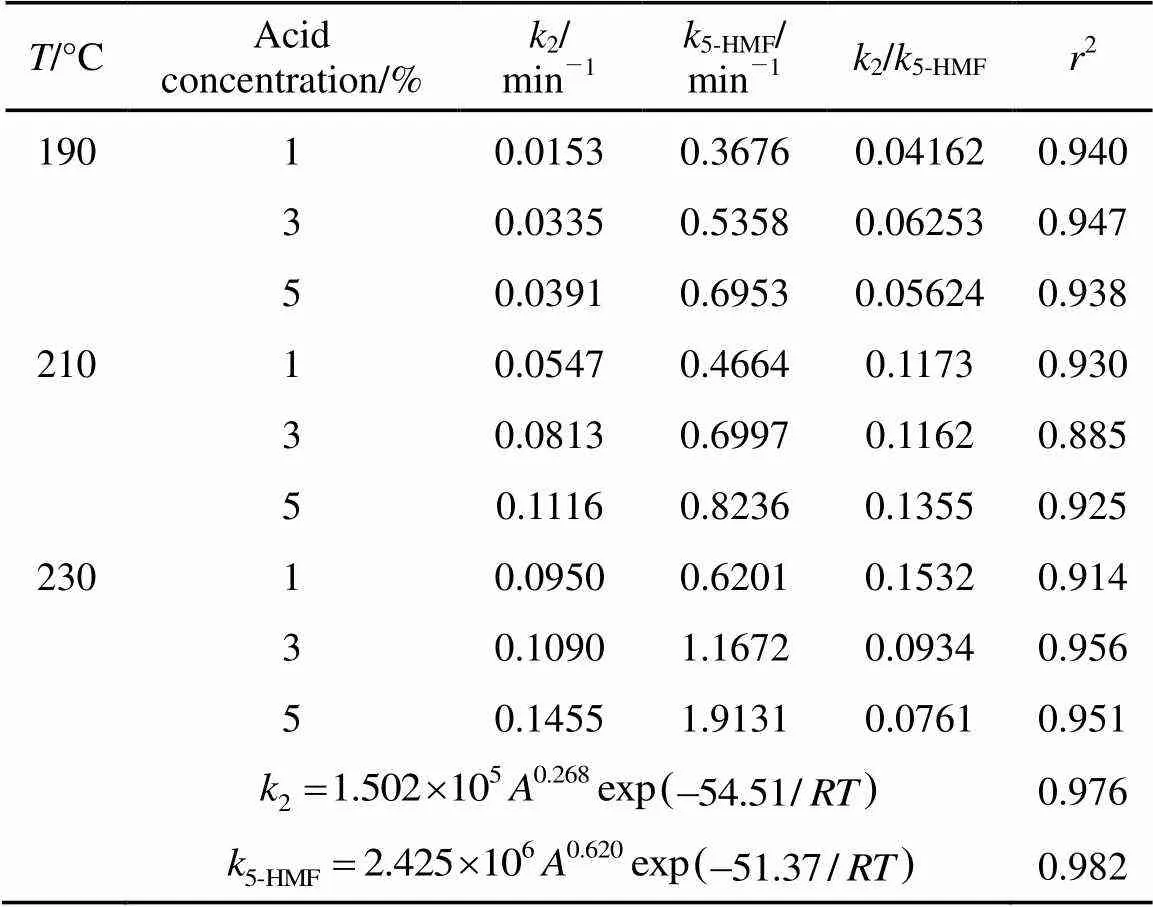
Table 2 Kinetic parameters of 5-HMF formation from wheat straw
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
- Simulation of Liquid Argon Flow along a Nanochannel: Effect of Applied Force*
