Current status of early gastric cancer screening research
Can Hu, Li Yuan, Xiangdong Cheng
1Department of Gastric surgery, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, Zhejiang 310022, China; 2Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; 3Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China
Gastric cancer is a predominant threat to the health and well-being of China’s residents.Data from the World Health Organization (WHO) in 2020 revealed that gastric cancer in China notably accounted for 44.0% of new cases worldwide and 48.6% of global deaths attributed to this malignancy1.In China, gastric cancer is the fourth most prevalent malignancy in terms of incidence among malignant tumors and the third leading cause of mortality2.This dire scenario poses a profound threat to health and longevity, and thus poses a substantial and pressing public health concern in China.The prevention and control of gastric cancer is thus a formidable challenge in the broader landscape of controlling malignant tumors in China.In this perspective, we discuss current methods of diagnosis and screening for gastric cancer (Figure 1).
Current status of gastric cancer screening
Japan and Korea, regions with high incidence of gastric cancer (GC), have initiated early GC screening research and have already achieved substantial progress.The diagnosis and treatment rates for early GC in those countries have reached 70%and 50%, respectively3.Therefore, despite Japan and Korea’s elevated GC incidence, their ratio of mortality to incidence is significantly lower than that in China and Western countries,as a result of their national screening strategies.In 2016, the Japanese government officially decided to include endoscopic screening for GC, targeting the population of individuals 40 years or older.However, because of the uneven distribution of medical resources, endoscopic screening was initially limited to cities with relatively abundant medical resources.Conducting direct GC examinations for the target population in areas with limited medical resources still presents challenges4.In Korea,the national cancer screening program has offered biennial gastroscopy or barium meal screening opportunities for individuals 40 years or older since 20025.Since the implementation of this program, the GC screening rate in Korea increased from 12.7% in 2002 to 43.9% in 20126.In 2005, the Chinese Ministry of Health launched the Cancer Early Diagnosis and Treatment Program in rural areas with high incidence of malignant tumors, with a specific emphasis on GC.In 2012,the Urban Cancer Early Diagnosis and Treatment Program was formally integrated into the national major public health service initiatives.China initiated an upper digestive tract cancer screening program targeting rural residents 40-69 years of age and urban residents 45-74 years of age.Before the screening, a questionnaire assessment is conducted, and gastroscopy screening is subsequently performed for high-risk individuals.However, because of large population sizes and relatively limited healthcare resources, implementing large-scale GC screening through gastroscopy is currently not feasible for the entire population.Further research and optimization are necessary to develop more tailored and context-specific screening methods.
Traditional molecular markers and detection methods for GC
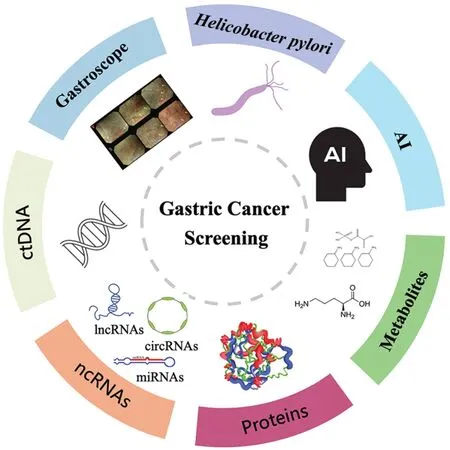
Figure 1 Current methods of gastric cancer screening.Current screening methods for gastric cancer include endoscopic screening, Helicobacter pylori detection, peripheral blood marker detection(ctDNA, ncRNAs, proteins, metabolites, etc.) and the application of artificial intelligence in medicine.
Currently, commonly used methods for GC screening include serum pepsinogen, serum gastrin, andHelicobacter pylori(Hp)antibody tests, as well as upper gastrointestinal barium meal examinations and endoscopic screening.Existing screening guidelines recommend GC screening for individuals ≥ 40 or 45 years of age who are at high risk.In addition, family history,diet, alcohol consumption, smoking status, and infection withHpor Epstein-Barr virus also increase the risk of GC development7.In China, the population at high risk of GC exceeds 300 million people.Given economic and healthcare resource optimization considerations, an urgent need exists to stratify the screening population by risk before gastroscopy, to enable the identification of high-risk individuals and increase screening efficiency.Therefore, specific GC screening markers must be determined, and an efficient screening model for high-risk individuals must urgently be established.
Hp
Hpis classified as a carcinogen in human GC by the International Agency for Research.Prospective studies have shown thatHpinfection increases the risk of progression to atypical hyperplasia or GC by 80%8.SerumHpantibody testing is cost-effective, rapid, and easily accepted by patients,and therefore is widely used in epidemiological investigations.Currently, in China’s early GC screening guidelines, the use of serumHpantibodies for initial screening of high-risk individuals for GC is recommended.However, serumHpantibody testing cannot distinguish between past infection and current infection, nor can it assess treatment efficacy after eradication.Individual genetic differences in human hosts also significantly affectHpantibody levels.
Gastrin-17 (G-17)
Multiple studies have indicated that elevated or diminished serum G-17 levels in patients with GC reflect the condition of gastric antral mucosal atrophy, thus serving as a potential early GC screening marker9.G-17 has relatively low sensitivity but acceptable specificity in diagnosing atrophic gastritis.When G-17 levels exceed 1.50 pmol/L, the risk of developing GC significantly increases.Because of the complexity of gastrin’s actions and its susceptibility to various influencing factors, use of G-17 as a standalone marker for GC screening is not recommended.
Pepsinogen (PG)
The incidence of GC is significantly higher in populations with diminished PG levels and PG I/II ratios, than in populations with stable levels.Moreover, among the GCs detected through screening, 90% are in early stages.A meta-analysis summarizing the accuracy of PG, based on data from 42 studies, has indicated a pooled sensitivity of 0.77 and specificity of 0.7310.Using serum PG as an initial screening tool followed by endoscopic examination enhances the detection rate of early-stage GC and precancerous lesions.Several countries, including Japan and Finland, have incorporated serum PG into their GC screening and prevention programs11.The most commonly referenced range for identifying individuals at high risk of GC is a PG I concentration ≤ 70 μg/L and a PG I/II ratio ≤ 3.0.However,serum PG levels can be influenced by regional, ethnic, dietary,infection, and environmental factors.Currently, no unified threshold exists for the serum PG levels used in GC screening,thus highlighting the need for national-level standardized criteria.Establishing these criteria will require further evidence from large-scale, multicenter, high-quality prospective studies.
Traditional GC screening methods
In 2011, Japan recommended the use of the serum PG andHpantibody combination method (ABC model) for GC screening.This approach significantly increases the detection rate of early-stage GC and can also identify GCs in individuals with serum PG negativity.In a large-scale opportunistic GC screening study, individuals have been categorized into risk groups A, B, C, and D, on the basis of the defined criteria in which PG I ≤ 70 μg/L and PGR ≤ 3.0 were considered PG positive; serum antibody titers ≥ 30 U/mL were consideredHppositive; and the risk of GC progressively increased from groups A to D12.Combined screening withHp, PG, and endoscopy significantly decreases screening costs with respect to those of endoscopy alone13.In addition, an expert consensus on early GC screening in China introduced a novel GC screening scoring model14, thereby providing a framework and direction for risk prediction models in high-risk populations.This scoring model integrates demographic factors and serum indicators for joint preliminary screening, including age, sex,PG, G-17, andHpantibodies, each of which is assigned a distinct score and is categorized into 3 levels.The efficacy of the GC screening score has been validated.However, before the novel GC screening scoring model is applied to large samples,further cohort studies and randomized controlled trials may be necessary to verify the reliability and stability of the results.Additionally, long-term observations of the model’s performance and benefits will be crucial.
Novel molecular markers and detection methods for GC
As tumor cells proliferate, they release nucleic acids such as proteins, metabolites, DNA, and RNA into the bloodstream.Therefore, circulating tumor DNA (ctDNA), microRNAs(miRNAs), long non-coding RNAs (lncRNAs), circular RNAs(circRNAs), and metabolites hold promise as non-invasive methods for early GC diagnosis.In comparison to traditional markers such as CEA and CA 19-9, these novel biomarkers offer enhanced sensitivity and specificity.Several pilot studies have demonstrated that ctDNA effectively distinguishes patients with GC from healthy individuals, with significantly higher sensitivity and specificity than conventional biomarkers15.Intriguingly, individuals with early stage disease, which is amenable to surgical resection, exhibit lower serum levels of ctDNA and fewer genetic alterations within the ctDNA itself.MiRNAs, a class of small non-coding RNAs, exhibit dysregulation in conditions preceding GC, such as atrophic gastritis, intestinal metaplasia, and early gastric developmental abnormalities.Specific miRNAs, including miRNA-21 and miR-376c, are upregulated in the serum in patients with early-stage GC, and have a positive predictive value as high as 90%16.So et al.have developed a clinical assay for detection of GC based on a 12-miRNA biomarker panel showing high performance in detecting GC17.Similarly, lncRNAs and circRNAs recently identified through RNA sequencing have been associated with tumor growth and metastasis.Levels of lncRNAs and circRNAs in the serum have been used not only to detect the presence of early stage GC, but also to monitor the extent of GC invasion and the presence of lymphatic metastasis16.Guo et al.have found that the lncRNA signature from circulating exosomes can be used as a biomarker for early detection of GC18.In our center, we have constructed a model based on plasma metabolic fingerprints for GC diagnosis, which has shown excellent diagnostic performance in both retrospective and prospective cohorts19.However, although liquid biopsies in the field of GC screening continue to advance, the challenges associated with their application should not be underestimated.Furthermore, liquid biopsy procedures must be standardized,to address complexities such as sample collection, target separation, and detection, while also providing reference materials.Moreover, the broad application of liquid biopsy early screening technology in clinical settings must also overcome 2 major problems: low accuracy and high sequencing cost.
Exploration of artificial intelligence in GC screening
Rapid development of artificial intelligence (AI) technolo gy has led to increasingly extensive applications in the field of medical image research.The recent integration of AI and deep learning into endoscopy procedures has yielded promising results showcasing AI’s potential to effectively aid in the detection of colorectal polyps, the prediction of Barrett’s neoplasia, and the enhancement of overall endoscopy quality.AI has been used to develop an endoscopy model that not only detects gastric mucosal lesions but also estimates the depth of lesion invasion20.In addition, we developed 3 types of tongue image-based AI deep learning diagnostic models, which adequately distinguish patients with GC from those without GC21.However, larger prospective studies are needed to validate these promising novel circulating molecules as reliable biomarkers for early GC.Moreover, as an emerging technology, deep learning harnesses convolutional neural networks to decipher the detailed patterns and presentations inherent in medical imaging data.Consequently, this method is invaluable in a multitude of complex clinical tasks by constructing models with intricate multiscale attributes.Currently, the key challenges associated with deep learning predominantly encompass issues such as data overfitting, the restricted “interpretability” of data, and the potential for suboptimal generalization.These challenges are intrinsically associated with the scale and diversity of the training datasets upon which deep learning relies.
Conclusions
Implementing early GC screening measures in the general population is a feasible and efficient approach to transforming the challenging landscape of GC diagnosis and treatment.At present, no unified high-quality risk prediction model is available for high-risk groups of individuals with GC worldwide.Gastroscope, which is considered the gold standard for GC diagnosis, has limitations-such as its invasive nature,relatively high cost, and low acceptance among the population-which make it impractical for large-scale GC screening.Therefore, effective strategies require the precise identification of individuals at high risk of GC or target populations for GC screening within the general population.Conducting gastroscopy in this selected group has been found to be a practical and efficient method.Additionally, advancements in the development of novel biomarkers and the use of AI have potential for achieving earlier enhanced detection of GC.
Grant support
This study was supported by The National Key Research and Development Program of China (2021YFA0910100), Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer (JBZX-202006), Medical Science and Technology Project of Zhejiang Province (WKJ-ZJ-2202 and WKJ-ZJ-2104),the National Natural Science Foundation of China (82074245,81973634, and 81903842), Natural Science Foundation of Zhejiang Province (LR21H280001), Science and Technology Projects of Zhejiang Province (2019C03049), and Program of Zhejiang Provincial TCM Sci-tech Plan (2018ZY006 and 2020ZZ005).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Xiangdong Cheng.Collected the data: Can Hu, Li Yuan.
Wrote the paper: Can Hu, Li Yuan.
 Cancer Biology & Medicine2024年3期
Cancer Biology & Medicine2024年3期
- Cancer Biology & Medicine的其它文章
- Erratum to Treatment strategies for patients with HER2-positive gastric cancer
- DNA damage response-related immune activation signature predicts the response to immune checkpoint inhibitors: from gastrointestinal cancer analysis to pan-cancer validation
- Bronchoalveolar lavage fluid assessment facilitates precision medicine for lung cancer
- A retrospective analysis of mature T- and NK-cell lymphomas
- Cervical cancer prevention in China: where are we now,and what’s next?
- Reducing the global cancer burden with gastrointestinal screening: China’s 30 years practice
