Multifunctional glycolipids as multi-targeting therapeutics for neural regeneration
Yutaka Itokazu
Many patients with neurodegenerative diseases,such as Alzheimer’s (AD) and Parkinson’s (PD) diseases suffer from disease progression without any satisfying clinical intervention,likely due to our lack of knowledge on how normal aging impacts the pathogenic mechanisms of these debilitating diseases.A growing body of literature has emerged in recent years that clearly demonstrates the involvement of glycolipids in the protein-oligomerization of neurodegenerative disorders.We hypothesize that changes in glycolipids composition are a common mechanism underlying the shift from healthy brain aging to the neuropathological processes of neurodegenerative diseases.
To effectively treat AD and PD,it is necessary to eliminate cytotoxic oligomers of amyloid-beta peptides (Aβs) and alpha-synuclein (α-syn) and sustain the functional neurons.Based on our mouse study,we propose to reduce neurotoxic oligomers of amyloid β-proteins (Aβ) and α-syn and to sustain functional neurons in the brains of patients by utilizing sugar-containing molecules called gangliosides as a strategy for neurodegenerative diseases.Since gangliosides are important modulators of proteins on plasma membranes,cell nuclei,mitochondria,and intracellular organelles,they are expected to modulate a variety of lipid microdomains to restore their physiological function in the brains of those living with neurodegenerative diseases.
Limited endogenous neurogenesis,spontaneously occurs following various attacks,such as ischemic stroke,traumatic brain injury,and Aβ,in an attempt to repair.However,the adult mammalian brain has a low ability to regenerate,making it difficult to fully recover the lost neurons and their functions.Our perspective is that an adequate molecular environment of glycolipid compositions are able to modulate membrane protein activities,such as growth factor receptors and/or to remodel chromatin structures to enhance gene expressions for regulating stemness and differentiation of stem/progenitor cells to achieve functional repair of damaged tissue.Proper glycolipid components (e.g.,GM1 and GD3) are conducive to regeneration.
The structural diversity and localization of cell surface glycolipids in glycolipid-enriched microdomains (also called lipid rafts or glycosynapses) place them in an ideal environment from which they can mediate processes such as intercellular interactions,adhesion,receptor function,and signaling.Glycolipid composition is dynamically changed during neural differentiation,e.g.,ganglioside GD3 is expressed in stem-progenitor cells (early stage) on the other hand,ganglioside GM1 is expressed in neurons (late stage of differentiation),thereby regulating distinct stages of differentiation.Our published and preliminary data demonstrated that GD3 maintains neural stem cell (NSC) characteristics and that GM1 epigenetically promotes neuronal differentiation (Wang and Yu,2013;Tsai and Yu,2014;Wang et al.,2014;Tsai et al.,2016;Tang et al.,2021;Fuchigami et al.,2023a,2023b;Itokazu et al.,2023;Itokazu and Yu,2023).
Cells as well as their subcellular organelles are surrounded by biological lipid membranes that define their individual cellular shape and maintain cellular organization.These semi-permeable membranes are essential for creating the chemical and electrical gradients needed for cellular functions.Recently,membrane lipids have been revealed to be important not only for separating the cellular and the extracellular environments but also for providing functional platforms for cellular signalings.We and others demonstrated that specific gangliosides control membrane functions of biological membranes,including the plasma,mitochondrial,and nuclear membranes.Signaling of neurotrophic factors is regulated by gangliosides,e.g.,GD3 for epidermal growth factor (Wang and Yu,2013) and GM1 for glial cell-derived neurotrophic factor (GDNF) (Hadaczek et al.,2015),which preferentially reside at the membrane microdomain of the plasma membrane.A key aspect for successful translational studies is that the functional activities of proteins and genes are highly dependent upon their molecular environments.An example is that a recent clinical trial of GDNF for PD failed,as have all previously published trials (Manfredsson et al.,2020).Since the expression of GDNF receptors is regulated by GM1,it is likely that molecular environment (e.g.,GM1 microdomain) is needed for the activation of further signaling.Therefore,in my opinion,GDNF alone (without GM1) could not activate the GDNF pathway.
Decreased or altered mitochondrial function is observed in neurodegenerative diseases (Simmons et al.,2020).Mitochondrial dysfunction would contribute to the etiopathology of these diseases.We showed that intranasally administered GD3 and GM1 dramatically restored levels of voltage-dependent anion channel 1,a major component of the outer mitochondrial membrane,known to regulate mitochondrial functions,to those of wild-type levels at substantia nigra and cortex in the human A53T α-syn-overexpressing PD model (A53T) mouse brain (Itokazu et al.,2021,2023).This result suggests that intranasal infusion of gangliosides can be an effective method to recover mitochondrial activities in neurodegenerative diseases.Further,we found that GD3 regulates mitochondrial dynamics by mediating the turnover of mitochondrial dynamin-related protein-1 (Tang et al.,2021).We also found that GM1 binds to other mitochondrial proteins to regulate mitochondrial functions.Targeting mitochondrial dysfunction with functional gangliosides is another promising approach for the development of future therapies for neurodegenerative diseases.
With regard to nuclear gangliosides,GM1 is involved in Ca2+homeostasis and nuclear GM1 was proposed to function as a modulator of nuclear Ca2+during axonogenesis (Ledeen and Wu,2015).We discovered that nuclear GM1 promotes neuronal differentiation by an epigenetic regulatory mechanism (Tsai and Yu,2014;Tsai et al.,2016).Nuclear GM1 binds with acetylated histones on the promoters of GM2 synthase (GM2S;N-acetylgalactosaminyltransferase (GalNAcT)),a critical enzyme for GM1 synthesis,as well as on theNeuroD1genes in differentiating neurons.Acetylation of histones H3 and H4 on theGM2Sgene promoter leads to the recruitment of trans-activation factors specificity protein 1 and activating protein 2 during neuronal differentiation.In addition,epigenetic activation of theGM2Sgene was detected as accompanied by an apparent induction of neuronal differentiation in NSCs responding to an exogenous supplement of GM1.GM1 is indeed localized in the nucleus where it can interact with transcriptionally active histones.Further,intranasal GM1 infusion increased nuclear expression of nuclear receptor-related 1,an essential transcription factor for differentiation,maturation,and maintenance of midbrain dopaminergic neurons in A53T (Itokazu et al.,2021) and GM2S-knockout (KO) mice.Further investigation revealed that GM1 induces epigenetic activation of the tyrosine hydroxylase (TH) gene,including augmentation of acetylated histones and recruitment of nuclear receptor-related 1 to theTHpromoter region in Neuro 2a cells.
PD reduces patients’ quality of life by the relentless progression of motor and non-motor symptoms.GM1 has been reported to be the key molecule for the pathogenesis of PD since GM1 binds to α-syn with high affinity to stabilize the alpha-helical state and inhibit oligomerization.Further,GM1-deficient mice exhibit PD-like movement disorders with reduced TH expression (Wu et al.,2011).The aged GM1-depleted mice are reported to have PD symptoms,including motor impairment,striatal dopamine depletion,gastrointestinal dysfunction,cardiac pathology,and cognitive impairment,suggesting that the deficiency of GM1 neuropathologically correlates with motor-and non-motor symptoms of PD in mice and humans.One of the non-motor symptoms is the neurogenic bladder which causes urinary tract infection,resulting in delirium,functional decline,falls,and hospitalization.GM1 deficient mice showed dysregulation of the bladder and ganglioside treatment restored bladder dysfunction in diabetic rats.The significance of our strategy is that multifunctional gangliosides modulate lipid microdomains to restore functions on the plasma,mitochondrial,and nuclear membranes.Based on rodent studies,the versatile nature of gangliosides has the potential to ameliorate several pathological features of PD,such as neurotoxic α-syn,GDNF signal inactivation,mitochondrial dysfunction,aberrant dopaminergic gene regulation,altered NSC activities,and other symptoms.
The toxic oligomers of Aβ and α-syn are the major pathologic hallmarks of AD and PD.Several studies showed that GM1 inhibits oligomerization of Aβ as well as α-syn,and deficiency of GM1 increases their accumulation,while the role of gangliosides in the pathogenesis of neurodegenerative diseases is not settled.Since major brain-type gangliosides,including GM1,are significantly decreased in patients with AD,PD,and Huntington’s diseases (Magistretti et al.,2019),to correct ganglioside compositions is a potential strategy to target common mechanism to treat neurodegenerative diseases.In our exciting data,nasal administration of gangliosides GM1 and/or GD3 reduced the neurotoxic α-syn level,and GM1 restored functional dopaminergic neurons at substantia nigra and striatum in A53T mice (Itokazu et al.,2021,2023).Furthermore,intranasal GM1 and GD3 restored mitochondrial functions and neurogenesis at the subventricular zone/olfactory bulb and dentate gyrus in the A53T mice (Fuchigami et al.,2023b).
It is desirable to develop more effective strategies to cure AD and PD.Although gangliosides have been explored as potential therapeutics in PD,for GM1 to be a viable therapeutic for PD,a new strategy of GM1 to be delivered to the brain through noninjection routes of administration will be necessary (Schneider,2022).The current problem is the lack of efficient routes for administering GM1 in a manner that permits it to efficiently cross the blood-brain barrier.Although with limited delivery,intravenous or intramuscular administration of GM1 to patients with stroke or spinal cord injury was found to be safe,with no adverse effects,and most importantly the patients showed neurological improvement.Schneider’s clinical trial (summarized in Schneider,2022) of subcutaneously injected GM1 showed beneficial effects on motor function and improved PET-based striatal dopamine transporter imaging in patients with PD.It was reported that less than 0.4% of an intravenously injected fluorinated ganglioside derivative,F-GM1,could enter the non-human primate (monkey) brain.We successfully developed a more convenient non-invasive delivery procedure by intranasal infusion of gangliosides.Intranasal ganglioside therapy is expected to slow disease progression and improve the quality of life of patients with PD.As the functional activities of proteins and genes depend on their molecular environments,such as ganglioside microdomains,multifunctional ganglioside can modulate protein and gene activities on plasma,mitochondrial,and nuclear membranes,in theory,may restore functions of specific molecules in the brains of patients.
The functional roles of gangliosides have been studied utilizing glycosyltransferase null mice that are global ganglioside KO mice.These global KO mice cannot address if gangliosides regulate NSC function in the postnatal stage,since ganglioside loss occurs before birth.We found that inducible GD3 deletion in postnatal radial glia-like NSCs promotes acute NSC activation,resulting in the loss of the longterm maintenance of the adult NSC pools in the mouse brain (Fuchigami et al.,2023a).The reduced neurogenesis in the subventricular zone and the dentate gyrus of GD3S-conditional-KO mice led to olfactory and memory dysfunctions.Thus,our results provide convincing evidence that postnatal GD3 is crucial to maintain the efficient pools of radial glia-like NSCs in the adult NSC niche.Although the biological significance of adult neurogenesis in humans is still under debate,the detection of adult neurogenesis in certain brain regions supports the author’s and others’ hypothesis that the adult brain exhibits more plasticity than previously thought,and this affects memory and the pathogenesis of neurodegenerative diseases.Emerging evidence from AD patients and mouse models supports an active level of neurogenesis in the early/moderate onset of the disease,which then becomes sluggish in the brains of patients with late/severe AD.Diminished neurogenesis occurs in normal aging,and it is hypothesized that an accelerated loss of the NSC pool is one mechanism that may contribute to a neurodegenerative brain.Impaired adult hippocampal neurogenesis occurs before signs of cognitive and memory deficits in AD.Therefore,sustaining endogenous neurogenesis has been suggested as an important target for treatment and prevention of neurodegenerative diseases.In theory,intranasal GD3 supplementation may amplify the numbers of NSCs in the subventricular zone and hippocampus in humans to help replenish brain circuits with neurons and improve the olfaction and memory functions with neurodegenerative and psychiatric diseases.In early to moderate stages of neurodegenerative diseases,in my opinion,GM1 would be sufficient to maintain neuronal functions.On the other hand,at more severe stages of diseases,first GD3 is needed to amplify NSCs and then GM1 to support neuronal differentiation of NSCs.Cell surface and intracellular glycolipid microdomains including GM1 or GD3 microdomains will provide additional clues underlying the neuroprotections and adult neurogenesis,which may be useful in developing novel strategies for disease treatment and neuronal regeneration (Figure 1).
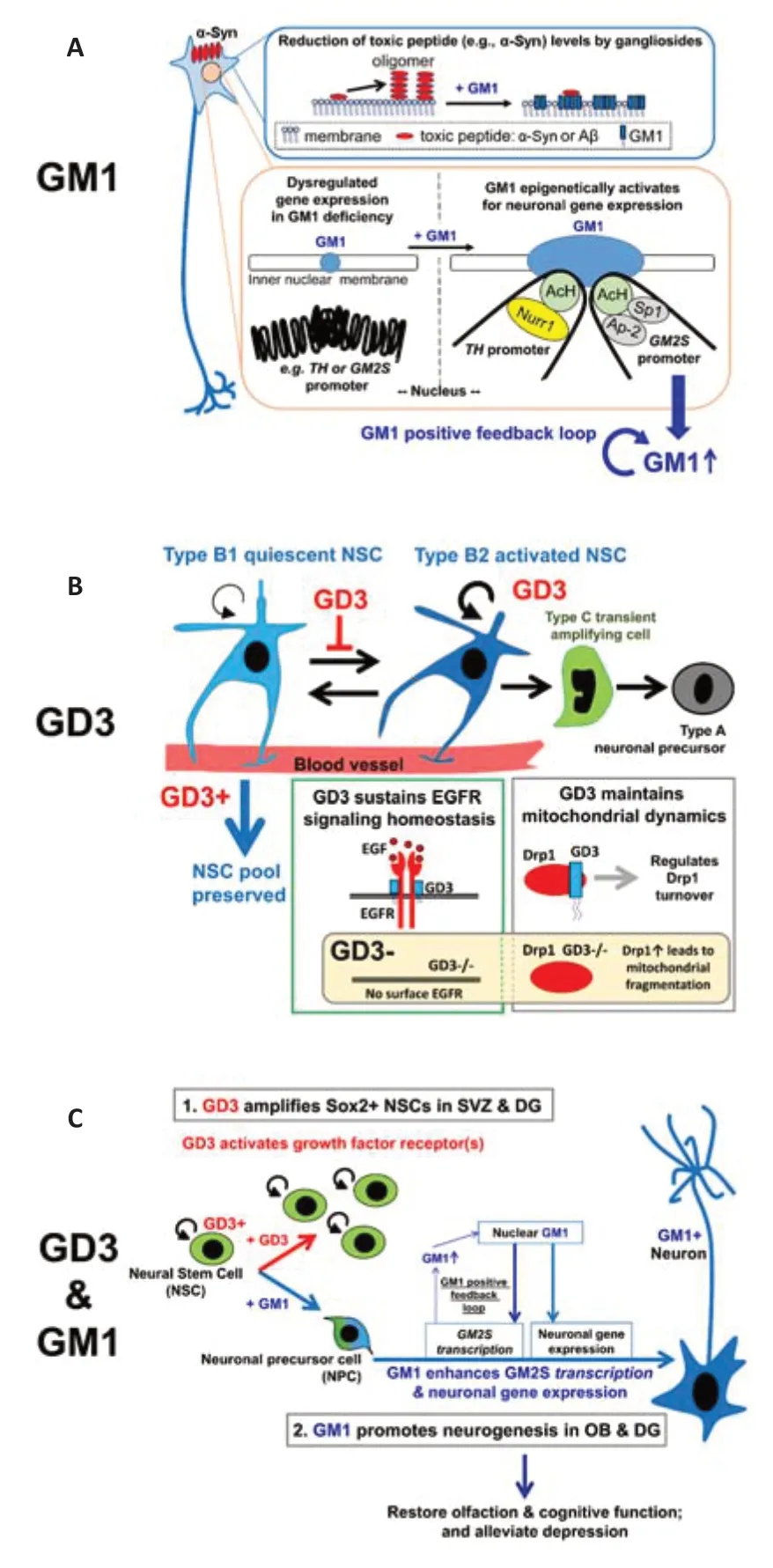
Figure 1|Mechanism of nasal ganglioside therapy.
This work is dedicated to the memory of Robert K.Yu (Bob Yu).
The present work was supported by a National Institute of Neurological Disorders and Stroke grant (R01 NS100839),a Sheffield Memorial Grant of the CSRA Parkinson’s Disease Support Group,and the excellent infrastructural support of the Department of Neuroscience and Regenerative Medicine,Medical College of Georgia at Augusta University (all to YI).
This article has been presented at a scientific meeting [American Society for Neurochemistry (ASN 2023 annual meeting)] on March 19,2023 in Lexington,USA.
Yutaka Itokazu*
Department of Neuroscience and Regenerative Medicine,Medical College of Georgia,Augusta University,Augusta,GA,USA
*Correspondence to:Yutaka Itokazu,PhD,yitokazu@augusta.edu.
https://orcid.org/0000-0001-7800-8262 (Yutaka Itokazu)
Date of submission:April 9,2023
Date of decision:May 25,2023
Date of acceptance:June 27,2023
Date of web publication:September 4,2023
https://doi.org/10.4103/1673-5374.382244
How to cite this article:Itokazu Y (2024) Multifunctional glycolipids as multi-targeting therapeutics for neural regeneration.Neural Regen Res 19(4):707-708.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Commentary on “Synchronized activity of sensory neurons initiates cortical synchrony in a model of neuropathic pain”
- A perspective on age-related changes in cell environment and risk of neurodegenerative diseases
- Anti-vascular endothelial growth factor drugs combined with laser photocoagulation maintain retinal ganglion cell integrity in patients with diabetic macular edema: study protocol for a prospective,non-randomized,controlled clinical trial
- STAT3 ameliorates truncated tau-induced cognitive deficits
- Multiple factors to assist human-derived induced pluripotent stem cells to efficiently differentiate into midbrain dopaminergic neurons
- Human umbilical cord mesenchymal stem cell-derived exosomes loaded into a composite conduit promote functional recovery after peripheral nerve injury in rats