Changes in Ribose and Ribokinase in STZ-induced Type 2 Diabetic Rat*
Dear Editor,
Type 2 diabetes mellitus (T2DM) is a metabolic disorder that impacts multiple organs including brain activity through mechanisms such as glucose toxicity,insulin resistance, mitochondrial dysfunction, and vascular damage[1-2]. As described by Su and his colleagues[3]in 2013, type 2 diabetics had abnormally high levels of urine ribose, suggesting that the patients suffered from not only glucose metabolism disorders,but also ribose metabolism disorders[4]. Previously, we employed streptozotocin (STZ)-induced type 1 diabetic rat and observed high ribose level in brain,which was associated with an abnormally lower level of transketolase (TKT), but not ribokinase (RK),phosphoribosyl pyrophosphate synthetase (PRPS),and glucose-6-phosphate dehydrogenase (G6PD)[5].Here, we used low-dose STZ-induced type 2 diabetic rats reared with high-fat diet (HFD) to study changes of ribose level in brain, and those of RK, TKT, PRPS,and G6PD which are directly and indirectly involved in ribose metabolism.
To clarify whether T2DM undergoes ribose metabolism disorders, we employed Sprague-Dawley(SD) rats (n=30) by a single intraperitoneal injection of STZ (35 mg/kg·bw), and fed them with a high-fat diet (HFD) for 18 weeks to establish STZ-induced T2DM animal model (T2DM group for short). Under the same conditions, rats fed with HFD without the injection of STZ (HFD group,n=9) and regular food(control group,n=10) were set as controls. Indices related to their blood lipids (Figure S1a), liver (Figure S1b) and kidney (Figure S1c, d) functions were shown, respectively. The body mass of STZ-induced rats grew over time but significantly lower than those in HFD and control group, starting from week 4 till week 18 (Figure 1a). STZ-induced rats suffered from high fasting blood glucose (FBG, (17±0.5) mmol/L)from week 4 to week 18, but this impact was not observed in HFD and control group (Figure 1b). The glycated serum protein (GSP) of STZ-induced rats was the highest level among the three groups (Figure 1c). These data were consistent with the characteristics of diabetes mellitus since a FBG level above 11.1 mmol/L is indicated diabetes[6]. To qualify the typical characteristics of the STZ-induced rat as a T2DM animal model, we also detected insulin,glucagon, and C-peptide in serum. Levels of serum insulin (Figure 1d) and glucagon (Figure 1e) except for C-peptide (Figure 1f) were markedly elevated in the STZ-induced rats compared with HFD and control group. High levels of FBG associated with high levels of insulin and glucagon in serum indicate insulin resistance[7]. Combining the above indicators, those changes are consistent with the type 2 diabetes model reference. Thus, we employed STZ-induced rats as type 2 diabetic animal model.
To elucidate whether ribose metabolism is changed in T2DM rats, we monitored urine ribose levels every 2 or 4 weeks by using the highperformance liquid chromatography (HPLC). While modeling, urine ribose levels of T2DM rats became significantly higher than those of HFD (n=9,P<0.01)and control (n=10,P<0.001) group (Figure 2a).Furthermore, T2DM rats showed the highest serum ribose level among the three groups after the 18-week administration (Figure 2b). Although the serum ribose level of the HFD group was significantly (n=9,P<0.05) elevated, the increase was distinctly (n=10,P<0.01) lower than that of the T2DM group. Moreover,levels of brain glucose and insulin in the T2DM rats also got minor but significant elevation compared to their control (Figure 2c, d), suggesting insulin resistance in the brain. According to the reports from Roshanravan and coworkers[8],N-methyl-D-aspartate(NMDA) receptors increase and present high levels in diabetes because of increasing glutamic acid[9-10].Similarly, our experimental results showed notably higher levels of NMNDR-2a and NMDAR-2b in the T2DM group compared with HFD and control group(Figure S2). These data demonstrated that STZinduced rats suffered from not only glucose but also D-ribose metabolic disorders.
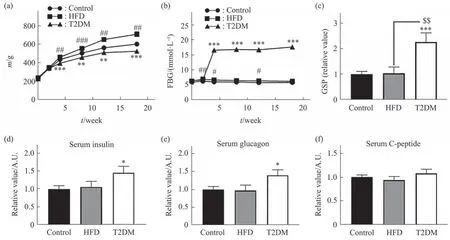
Fig. 1 Physical and serum measurements of STZ-induced rats for T2DM
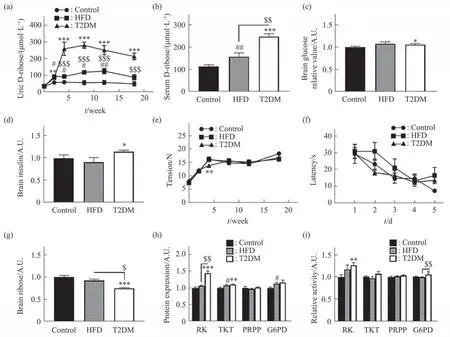
Fig. 2 The levels of ribose and related enzymes in T2DM rats
To compare STZ-induced T1DM rats which suffered markedly from learning deficiency[5], we measured the grip strength of the rats (P<0.05,Figure 2e) and then tested their spatial learning abilities of the three groups by using Morris water maze. In contrast to the T1DM rats, T2DM group showed no significant differences in the time of finding the platform as escape latency for each of the five training days, compared to HFD and control group (P<0.05, Figure 2f). By the way, in open field test, no significant behavioral changes were observed in the T2DM rats for time in the center (P>0.05,Figure S3a), number of stand (P>0.05, Figure S3c),number of grooming (P>0.05, Figure S3d) except for center square entries (P<0.05, Figure S3b) compared to control. However, STZ-induced T1DM rats showed strikingly changes in all behavioral indices based on the open field test[5].
We also checked whether ribose metabolism has changed in the brain of STZ-induced rats. As shown in Figure 2g, ribose levels in the brain of T2DM group were much lower than those of HFD (n=9,P<0.05)and control (n=10,P<0.001) group. To investigate the potential mechanism for significant reduction of ribose levels, we measured the expression levels of the enzymes RK, TKT, PRPP, and G6PD by using ELISA assay. Significantly, RK expression levels were elevated (n=10,P<0.001) in the brain of STZinduced rats (Figure 2h), and much higher than those in HFD (n=9,P<0.01) and control (n=10,P<0.001)group. Consistently, the RK activity (n=10,P<0.01) in T2DM group significantly increased, and so did G6PD (n=10,P<0.05) under the experimental conditions (Figure 2i). The TKT protein also increased in T2DM group, but not its activity.Notably, neither the expression nor the enzymic activity of PRPP was markedly changed among the three groups.
The STZ-induced T2DM group exhibited high levels of ribose and glucose in serum, which is similar to STZ-induced type 1 diabetic rat[5]and Goto-Kakizaki (GK) rat[11]. Clinical investigations also showed that patients with either T1DM or T2DM suffered from high levels of ribose in serum and urine[4-5,12]. The level of serum ribose in diabetic patients is positively correlated with that of glycated serum protein[13]. Those data in DM animal models support that type 1 and type 2 diabetes mellitus may have both ribose and glucose metabolism disorders.
As described by Yu and colleagues[6], STZinduced type 1 diabetic rat presents with high levels of ribose in serum and urine as well as brain.Accordingly, the expression and activity of TKT of the T1DM rats were significantly decreased in their brain. TKT participates in the pentose phosphate pathway (PPP), which is essential for energy transduction and ribose production for nucleic acid synthesis[14]. TKT deficiency leads to ribose accumulation in cells, which is indeed a mechanism in developing to the technique of ribose production[15].However, the mechanism why STZ-induced T2DM rats have high serum ribose levels and low brain ribose levels needs to be further elucidated.
Different from STZ-induced type 1 diabetic model, STZ-induced T2DM rats in current study showed a markedly low ribose level in their brain. To understand the underlying mechanisms, we propose the following possibilities. Firstly, the activity of TKT does not significantly change in the brain of STZinduced T2DM rats. Secondly, a marked elevation occurs in both the expression and activity of RK,which catalyzes the phosphorylation of ribose to ribose-5-phosphate (R-5-P), and triggers the first step in the metabolism of ribose[16]. RK prepares ribose for entry into the PPP and uses the sugar in synthesizing nucleotides and other compounds[17]. Thirdly, STZ induces neurotoxicity besides damage to pancreatic beta cells[18]. Compared to T2DM modeling, to induce T1DM requires double doses of STZ (70 mg/kg·bw)for the injection of rats. Thus, it was understandable that ribose metabolism differs from the two diabetic animal models. Finally, preparation of T2DM rat takes much longer time than that of T1DM rat, during which a compensatory effect on ribose metabolism might occur in the rat brain, including the improvement of RK activity.
Brain requires a continuous supply of energy in the form of ATP[19], and diabetes is involved in disturbances in brain energy metabolism since insulin resistance[20]. Ribose is the rate-limiting compound in producing energy molecules including ATP[21]. That is to say, increases in RK activity result in decrease of ribose levels in the T2DM rat brain and may provide more energy for brain activities. Therefore, RK may be used as a potential drug targeting the regulation of ribose metabolism. However, further investigation is necessary to reveal which pathway and what mechanism is involved in activating RK in STZinduced type 2 diabetic rats.
Acknowledgements We thank Ms. SHI Xiang and Mr. ZHOU Lei (Institute of Biophysics, Chinese Academy of Sciences) for their providing veterinary care, breeding, the management of laboratory animals and technical support.
Supplementary Available online (http://www.pibb.ac.cn or http://www.cnki.net):
PIBB_20230150_Figure_S1.pdf
PIBB_20230150_Figure_S2.pdf
PIBB_20230150_Figure_S3.pdf
PIBB_20230150_Doc_S1.pdf
KANG Nian-Xin1)**, YU Le-Xiang2)**, LIU Ying1),HE Rong-Qiao2)***
1)School of Life Sciences, Beijing University of Chinese Medicine, Beijing102401,China;
2)Institute of Biophysics, University of Chinese Academy of Sciences,Beijing100101,China
* This work was supported by grants from Beijing Brain Project(Z161100000217141) and the Fundamental Research Funds for the Central Universities (2020-JYB-ZDGG-051).
** These authors contributed equally to this work.
*** Corresponding author.
Tel: 86-13552534019, E-mail: herq@ibp.ac.cn
DOI: 10.16476/j.pibb.2023.0150
Received: April 13, 2023 Accepted: April 21, 2023

