Intercellular transmission of cGAS-STING signaling in cancer
Qirou Wu, Xiaohong Leng, Pinglong Xu
1MOE Laboratory of Biosystems Homeostasis and Protection and Zhejiang Provincial Key Laboratory for Cancer Molecular Cell Biology, Life Sciences Institute, Zhejiang University, Hangzhou 310058, China; 2Institute of Intelligent Medicine,Hangzhou Global Scientific and Technological Innovation Center, Zhejiang University (HIC-ZJU), Hangzhou 310058, China;3Cancer Center, Zhejiang University, Hangzhou 310058, China
Cellular communication is necessary for organizing multicellular organisms, and is critical in tumorigenesis and progression.Immune and tumor cells use the cGAS-STING mechanism to sense and respond to genomic instability,DNA damage, and mitochondrial dysfunction induced by extra- and intracellular stresses.As an essential component of innate nucleic acid sensing, cGAS-STING signaling is critical in various pathological processes, including microbial infection, autoimmunity, inflammation, organ degeneration, and malignancy.The cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS) senses aberrant or displaced dsDNA in the cytosol and synthesizes 2′3′-cyclic GMP-AMP(cGAMP).Endoplasmic reticulum (ER)-resident adaptor stimulator of interferon genes (STING), mobilized by this second messenger, is trafficked among the ER, ER-Golgi intermediate compartment, and Golgi apparatus, and forms distinct STING signalosomes, which in turn trigger various molecular programs controlling mRNA translation, autophagy, organelle reorganization, and interferon(IFN) production1.The non-canonical functions of cGASSTING signaling have recently gained extensive attention.These functions occur in various cellular processes, including senescence, autophagy, and cap-dependent mRNA translation1,2.
Notably, the complexity of cGAS-STING signaling is further enhanced by an intriguing feature of intercellular transmission (Figure 1) that has recently been established in several seminal discoveries.The effective intercellular transfer of cGAS-STING signaling enables the sharing of critical information arising from infected, injured, or cancerous cells and the activation of multiple bystander cells in innate immune responses.In cancers, intercellular communication of cGASSTING signaling connects the tumor microenvironment and pathogenic, immune, and stromal cells, thus promoting or compromising various immune responses and substantially altering disease progression.Understanding this intercellular interplay would greatly aid in developing therapeutics against malignancy.In this perspective, we discuss current knowledge,effects on cancer biology, and implications of in cancer progression and therapeutics.
Intercellular transmission of dsDNA
Unlike normal cells, free cytoplasmic dsDNA commonly arises in tumor cells because of chromatin instability and altered metabolism.These dsDNAs are derived from multiple sources,including genomic, mitochondrial, apoptotic, and transposable elements, all of which activate cGAS-STING signaling and subsequently induce innate immune responses3.The modes of dsDNA transmission include phagocytosis, facilitated internalization, and extracellular vesicles (EVs).
Phagocytosis
dsDNA transfer between cancer cells and immune cells typically occurs through phagocytosis in the tumor microenvironment.Tumor cell-derived DNA accumulates in the cytoplasm of phagocytes, such as dendritic cells and macrophages, and triggers the activation of cGAS-STING signaling, thus facilitating antigen presentation and promoting acquired immune responses4.
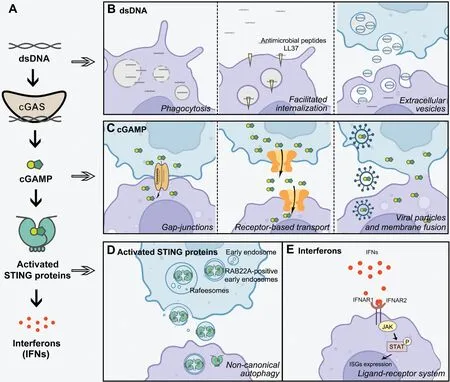
Figure 1 Intercellular communication in cGAS-STING signaling.(A) Schematic diagram of canonical cGAS-STING signaling.The DNA sensor cyclic GMP-AMP synthase (cGAS) recognizes a wide range of double-strand DNA (dsDNA) and is activated, thus generating the second messenger 2′3′-cyclic GMP-AMP (cGAMP), which in turn activates membrane-bound stimulator of interferon genes (STING) and induces interferons (IFNs).(B-E) Intercellular transmission of cGAS-STING signaling, including the transfer of dsDNA through phagocytosis, facilitated internalization, and extracellular vesicles (B); the intercellular transmission of cGAMP through gap junctions, receptor-based transport,viral particles, and membrane fusion (C); the intercellular transmission of activated STING triggered by RAB22A-mediated non-canonical autophagy (D); the intercellular communication of IFN signaling through autocrine and paracrine ligand-receptor systems, and subsequent activation of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway and induction of the expression of various interferon-stimulated genes (ISGs) (E).
Facilitated internalization
High levels of dsDNA are released into extracellular space after cell death.This DNA is typically non-immunogenic because of its rapid degradation.However, some antimicrobial peptides, such as LL37, bind extracellular dsDNA and facilitate its internalization into monocytes, which produce type I IFNs5.
Extracellular vesicles
dsDNA associated with small EVs (<200 nm in diameter) has been found to mediate intercellular communication in scenarios including infection, inflammation, and malignancy.EVs are lipid bilayer-bound vesicles in biological fluids and are released and captured by cells.EV-mediated delivery of foreign DNA to bystander cells is frequently detected in pathogen infections6.Tumor cell-derived EVs with dsDNA cargo regulate the tumor microenvironment, thereby promoting tumor progression and metastasis.In Crohn’s disease, EVs carrying dsDNA are shed from sites of inflammation and subsequently activate cGAS-STING signaling and enhance innate immune responses in macrophages, thus aggravating the disease7.Upregulation of the mitochondrial DNA-binding protein Lon also induces the secretion of EVs with mtDNA and PD-L1,thereby further inducing the production of IFN and IL-6,and attenuating T-cell immunity.Because dsDNA is found in many stages of cancer and has the potential to strengthen cancer immunity and mediate metastasis, further exploration of its generation and transmission is expected to yield exciting findings in cancer biology.
Intercellular transmission of cGAMP
cGAMP is secreted by cancer cells into the extracellular space and subsequently activates immune responses in receptor cells through various paracrine pathways.Transfer of cGAS-synthesized cGAMP to bystander cells is a primary mechanism underlying cytokine-independent activation of innate and adaptive immune responses.The extracellular hydrolase ENPP1 degrades extracellular cGAMP, thereby controlling the levels of cGAMP in the extracellular environment8.
Gap junctions
Cells transfer cGAMP to neighboring immune cells and activate the immune response through gap junctions, intercellular structures consisting of arrays of intercellular channels in the plasma membrane that allow for the direct transfer of ions and small molecules between cells.Connexins form gap junctions in vertebrates, and transmission of cGAMP relies on the expression of connexins.For instance, connexins 43 and 45 enable the transfer of cGAMP between epithelial and macrophages9.This macrophage transactivation amplifies positive-feedback loops of antitumor immune responses.However, cGAMP transfer also promotes tumor growth and metastasis.Brain metastatic cancer cells use gap junctions to transfer cGAMP into astrocytes in a process mediated by protocadherin 7 and connexin 43, thus resulting in the propagation of cytokines that support tumor growth, such as interferon-α (IFN-α) and tumor necrosis factor (TNF)10.In this scenario, the modulators of gap junctions that break this paracrine loop may serve as targets to treat established brain metastasis.
Receptor-based cGAMP transport
Unlike gap junctions, receptor-based transport facilitates the transmission of cGAMP to distant cells.The volume-regulated anion channel (VRAC) mediates cGAMP import and export depending on the cGAMP chemical gradient.Knockdown of leucine-rich repeat containing 8 (LRRC8A) has been found to eliminate VRAC activity and to inhibit 50%–70% of cGAMP uptake in various primary and cultured cells11.Other cGAMP transporters, including solute carrier family 19 member 1(SLC19A1) and SLC46A2, have been found to have roles in cells such as THP-1, Nu-DUL-1, U937, and CD14+monocytes,according to two recent studies12,13.ATP-gated P2X purinergic receptor 7 (P2X7R) is another protein that may potentially transport cGAMP14.Opening the ATP-gated P2X7R channel requires a high concentration of ATP, which may be present during cell damage or death, thus allowing nanometer-sized molecules such as cGAMP to pass through.Therefore, receptor-based transport of cGAMP requires various yet-to-beidentified transporters and functions in a context-dependent manner.
Viral particles and membrane fusion
Expressing HIV-1 Env in donor T cells leads to a membrane fusion reaction with uninfected CD4+macrophages.The intercellular transfer of cGAMP through fusion sites augments type I IFN responses in macrophages15.HIV-1 also rapidly transfers cGAMP to surrounding cells through viral particles, as validated by mass spectrometry analysis.These viral particles containing cGAMP stimulate robust STING-mediated immune responses in cGAS-deleted dendritic cells16,17.However, the role of cGAMP transmission by viral particles and membrane fusion is less understood in cancer biology.
Intercellular transmission of activated STING proteins
STING is an ER-resident transmembrane protein that traffics to the Golgi after activation, and is degraded through autophagy and lysosomes.The lifespan of STING is associated with the membrane system.Notably, the Kang group18has recently revealed that activated STING proteins are packaged into RAB22A-induced EVs and transported intercellularly through non- canonical autophagy.EVs containing activated STING induce downstream effects of cGAS-STING signaling in recipient cells.Host-derived STING proteins have been detected in exosomes isolated from HSV-1 infected cells, although no secretion signal peptide in STING has been identified.Activated STING proteins are packaged into Rafeesomes, the organelle formed by the fusion of ER-derived and RAB22Amediated non- canonical autophagosomes with RAB22Apositive early endosomes18.During the process, RAB22A inactivates RAB7, thereby suppressing the fusion of the Rafeesome with the lysosome, and enabling the secretion of these inner vesicles containing activated STING as EVs.These STINGcontaining EVs induce IFN-β release from recipient cells into the tumor microenvironment, thus promoting antitumor immunity.These intriguing observations provide a new perspective regarding the spread of STING-armed immunity and alternative forms of intercellular transmission of functional membrane proteins.
Intercellular transmission of interferons
IFNs have various functions in immunity and diseases, such as adaptive immune regulation, pathogen defense, antitumor immunity, and autoimmune responses19.Type I and III IFNs are crucial secretory proteins downstream of Toll-like receptors, RIG-I-like receptors, and cGAS that activate the JAKSTAT pathway in a paracrine manner by binding receptors of bystander cells such as IFNAR1 and IFNAR2.This ligand-receptor-based intercellular communication of IFNs promotes the generation of hundreds of interferon stimulated genes,thus affecting and modulating IFN signaling19.Intercellular transmission of IFN signals in cancer commonly occurs and is crucial for modulating tumorigenesis and cancer progression,such as through directly prolonging the cell cycle and inducing apoptosis.IFNs restrain tumor metastasis by affecting vascular endothelial cells and increasing antitumor immunity in immune cells3, such as through activating dendritic cells and cytotoxic CD8+T cells.Consequently, IFN-β is a potential adjuvant for cancer therapy20.However, the effects of IFNs on cancer biology are complex, and the protumorigenic effects of IFNs are also well accepted.
Conclusions
In most scenarios, the activation of cGAS-STING signaling facilitates antitumor immunity; consequently, leveraging theintercellular transmission of cGAS-STING signaling might serve as a promising target for treating cancers.The intercellular transmission of cGAS-STING signaling might be targeted,probably context-dependent, to benefit disease therapeutics(Table 1).For example, the physiological concentration of cGAMP is controlled by the cGAMP hydrolase ENPP1, which serves as a potential target for leveraging cGAS-STING transmission.A potent membrane-impermeable ENPP1 inhibitor,compound 32, has been found to enhance plasma cGAMP concentrations and alleviate tumor burden21significantly and has been studied in a clinical trial22.Furthermore, DNAcontaining EVs secreted from topotecan-treated cancer cells have been found to activate dendritic cells and to facilitate antitumor immunityviacGAS-STING signaling.In contrast,pharmacological inhibition of gap junctions hinders the transfer of cGAMP from cancer cells to astrocytes and restricts brain metastasis10.The delivery of activated STINGviaEVs adds another layer for leveraging cGAS-STING signaling by inducing the production of IFN-β and facilitating antitumor immunity18.However, activators and inhibitors of cGAMP transporters, such as LRRC8A, remain to be developed and examined.
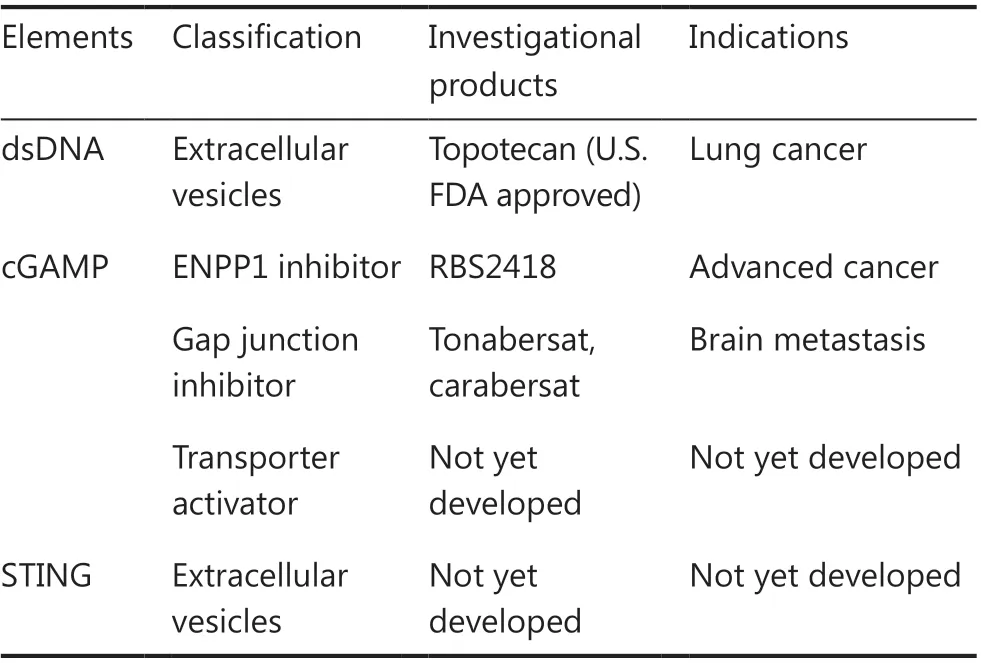
Table 1 Clinical trials targeting the intercellular transmission of cGAS-STING signaling in cancers
The beneficial roles of cGAS-STING signaling in cancer progression have recently gained considerable attention.Other critical questions, such as how multiple innate DNA sensors activate the context-dependent response programs and crosstalk with each other and how the cGAS-STING pathway communicates with cancer-specific metabolism and nutrients, are intriguing questions remaining to be answered.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2023年2期
Cancer Biology & Medicine2023年2期
- Cancer Biology & Medicine的其它文章
- Optimization of regional nodal irradiation in the era of sentinel lymph node biopsy
- Association between homologous recombination deficiency and outcomes with platinum and platinum-free chemotherapy in patients with triple-negative breast cancer
- Cancer risk in relatives of BRCA1/2 pathogenic variant carriers in a large series of unselected patients with breast cancer
- Circular RNAs: implications of signaling pathways and bioinformatics in human cancer
- Conceptualizing the complexity of ferroptosis to treat triplenegative breast cancer: theory-to-practice
- 2022 Chinese expert consensus and guidelines on clinical management of toxicity in anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma
