Phylogenetic placement and diet of homalopsid snake Miralia alternans (Ruess, 1833)
DEAR EDITOR,
Miralia alternans(Ruess, 1833) is distributed in Borneo,Sumatra, and Java in Southeast Asia. The species is morphologically similar toRaclitiaindica, a monotypic genus known from Peninsular Malaysia. Here, we collected a juvenile specimen ofM. alternansfrom Borneo, and report on its coloration in life and first prey item recovered from the species. We also explored the phylogenetic position of the genus using molecular phylogenetic analysis of mitochondrial DNA (mtDNA) gene cytochromeb(cytb) and nuclear gene prolactin receptor (PRLR), confirmingM. alternansas sister toR. indica, with the two genera exhibiting relatively high genetic divergence in cytb(13.0%-13.1%).
Mud snakes (Homalopsidae), which include 56 species in 29 genera, are primarily distributed in Southeast Asia (Uetz et al., 2022).EnhydrisSonnini & Latreille, 1802, the formerly largest genus in the family, was divided into 15 genera by Murphy & Voris (2014). While several recent studies have inferred the phylogenetic relationships within the family, the positions of certain genera were not investigated, as half of the genera are only known from museum specimens(Bernstein et al., 2021). The monotypic genusMiraliaGray,1842 was previously considered a synonym ofEnhydris(e.g.,Gyi, 1970), but later resurrected by Murphy & Voris (2014).Several studies have identified morphological similarities betweenMiralia alternansandRaclitia indica, suggesting they may be closely related (Gyi, 1970). However, molecular analysis has not yet been conducted due to the lack ofM.alternanstissues (Murphy, 2007; Quah et al., 2018). In 2010,we collected a juvenileM. alternanssnake in Kuching,Sarawak, Malaysian Borneo, with tissue from this specimen used to clarify its phylogenetic position.
TheM. alternansspecimen was collected during a field
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium,provided the original work is properly cited.
Copyright ?2022 Editorial Office of Zoological Research, Kunming Institute of Zoology, Chinese Academy of Sciences survey at the Matang Wildlife Centre, Kuching, Sarawak,Malaysia, in August 2010. Tissue samples were taken for genetic analyses and deposited with the voucher specimen in the Sarawak Research Collection, Sarawak Forest Department (SRC). In addition to the new specimen, we examined the morphological characters of previously deposited specimens in the Museum Zoologicum Bogoriense(MZB), Sarawak Museum (SM), and Zoological Reference Collection of the Lee Kong Chian Natural History Museum at the National University of Singapore (ZRC). Scale terminology and measurements followed Murphy & Voris (2014) and Quah et al. (2018).
For molecular analyses, DNA was extracted and fragments of the mitochondrial gene cytb(1 053 bp) and nuclear genePRLR(573 bp) were amplified by polymerase chain reaction(PCR). The PCR products were sequenced with PCR primers and BigDye v3.1 using Sanger sequencing methods, and the obtained sequences were deposited in GenBank under accession numbers LC667473 (for cytb) and LC667474 (forPRLR). In addition to the newly sequenced data forM.alternans, we used Homalopsidae sequencing data from Bernstein et al. (2021) to identify the phylogenetic position ofM. alternansamong other homalopsid genera (Supplementary Table S1). Maximum-likelihood (ML) and Bayesian inference(BI) methods were used to conduct phylogenetic analyses.UncorrectedP-distances for the cytbregion among sequences were calculated. Details are provided in the Supplementary Materials and Methods.
The specimen (SRC 00064) was collected on 30 August 2010 in lowland primary forest at the Matang Wildlife Centre,Kuching, Sarawak, Malaysia. The specimen was found at 1930h under leaf litter, close to a small, shallow, sandybottomed stream (1-2 m wide). To date, only three specimens have been reported from Borneo (Gyi, 1970; Murphy, 2007;Murphy & Voris 2014). Thus, the new specimen represents the fourth record of the species from Borneo.
Scale counts ofM. alternansshowed variation in supralabials 7-8, with 3rd-4th, 4th, or 4th-5thbordering orbit,supralabial formula 2-2-3, 3-1-3, 3-2-2, 3-1-4, or 3-2-3, 1stor 2ndsupralabials contacting nasal cleft, infralabials 8-11,preoculars 0-1 (when no preocular prefrontals touching anterior side of orbit), postoculars 1-2, mid dorsal scale rows 19-20, ventrals 127-164 in males and 120-152 in females,and subcaudals 28-39 in males and 23-36 in females(Supplementary Tables S2, S3). In life, dorsum glossy dark purple brown with narrow, transverse orange bands about one dorsal scale in length; first band on occiput lighter in color than other bands, about three dorsal scales in length; venter glossy dark purple brown, with yellow white bands about two ventrals in length, most interrupted by dark areas on midline(Figure 1B, C, D). Color pattern was generally similar in all preserved specimens, but dorsal transverse bands were less clear and first band on occiput was shorter in adult specimens than in juvenile specimens.
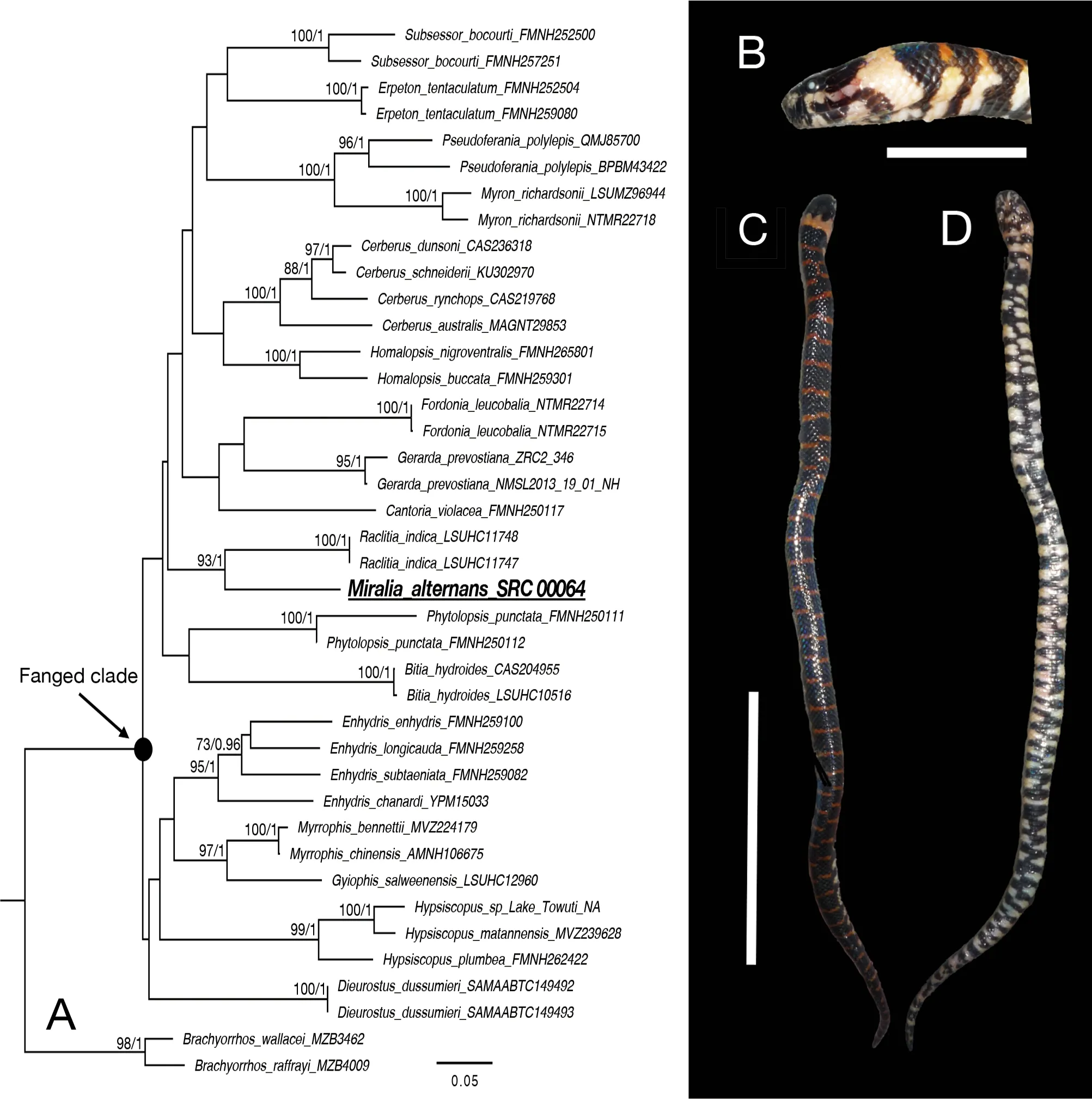
Figure 1 Phylogenetic relationships of Homalopsidae and Miralia alternans specimen
The phylogenetic tree topologies using ML and BI were identical, except that the basal lineage relationships were not strongly supported in both analyses. Therefore, we only present the ML tree (Figure 1A). Analysis indicated thatM.alternanswas nested within the fanged clade of Homalopsidae, although clade monophyly was not well supported. The sister relationship betweenM. alternansandR. indicawas strongly supported, with an uncorrectedPdistance of 13.0%-13.1% in cytbbetween the two species(Supplementary Table S4).
One examined specimen (MZB Ophi. 4877) was fixed as it was in the process of regurgitating an Asian swamp eel(Monopteruscf.albus) (Supplementary Figure S1).
The present study revealed huge morphological variation withinM. alternans(Supplementary Tables S2, S3), indicating that the species may include multiple cryptic species. We only used samples from Sarawak and Java for morphological examination and from Sarawak for molecular analysis. Thus,taxonomic assessment of the species using wider morphological and phylogenetical sampling is needed.
Phylogenetic analysis indicated and confirmed a sister relationship betweenM. alternansandR. indica, as inferred in previous studies based on morphology (Gyi, 1970; Murphy et al., 2011). AlthoughM. alternansandR. indicaare morphologically similar, their genetic distance based on the mitochondrial cytbgene was relatively high (13.0%-13.1%).Maximum interspecific genetic distances in cytbwithin a genus are about 9%-12% and minimum intergeneric genetic distances within a family are about 10%-14% (Supplementary Table S4), comparable to the genetic divergence betweenM.alternansandR. indica.
Gray (Gray, 1849) suggested the possibility thatM.alternansmay be a variety ofR. indica.Although the two species are morphologically similar, the number of ventrals generally differs (120-164 inM. alternansvs. 152-175 inR.indica), and the two species are deeply genetically divergent.Thus, we confirmed distinct species status for both species based on molecular and morphological differences.
However, the generic status of the two species may be reconsidered, as we could not find morphological differences betweenMiraliaandRaclitiacomparable to those of different genera. Both these genera were described in Gray (1842),and thusMiraliacould be treated as a junior synonym ofRaclitiaor vice versa.To determine their generic treatment,additional morphological data, such as maxillary teeth and hemipenial morphology, as well as genetic material, especially topotypicM. alternansfrom Java, are crucial.
Murphy et al. (2011) suggested thatM. alternansandR.indicamay be fossorial and vermivorous. We foundMonopteruscf.albus(Supplementary Figure S1) as a food item ofM. alternansfor the first time, and one specimen (SRC 00064) was collected very close to a stream. Therefore, we can infer thatM. alternansis at least partly aquatic and feeds on fish. However, more information on its natural history is required to reveal the dietary and behavioral habits of this rare species.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Field surveys and specimen collection were approved by the State Government of Sarawak (Research Permit No.NPW.907.4.2(III)-68).
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
I.F., T.K., and K.E. conceived and designed the study. I.F.performed the experiments, measured the specimens, and wrote the manuscript. M.M., K.N., and K.E. revised the manuscript. M.Y.H. arranged the field survey. M.Y.H., M.M.,K.N., and K.E. collected the specimens in the field. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The State Government of Sarawak kindly permitted us to conduct the project and the RDID provided facilities for conducting research and molecular experiments, including sequencing. We are grateful to R. ak S. Pungga, P. ak Meleng, and T. Itioka for their support in obtaining permission to conduct research. We thank A. Hamidy (MZB) and K.O.Chan (ZRC) for kindly providing access to specimens deposited in the collections of their respective institutions. We thank H. Ota for valuable comments and suggestions for identification.
Ibuki Fukuyama1,*, Tomonori Kodama2, Koshiro Eto3,Masafumi Matsui1, Mohamad Yazid Hossman4,Kanto Nishikawa1,51Graduate School of Human and Environmental Studies,Kyoto University,Yoshida-Nihonmatsu,Sakyo,Kyoto606-8501,Japan2Graduate School of Science,Kyoto University,Kitashirakawa-Oiwake,Sakyo,Kyoto606-8502,Japan
3Kitakyushu Museum of Natural History &Human History,Higashida 2-4-1,Yahatahigashi,Kitakyushu,Fukuoka805-0071,Japan4Research,Development,and Innovation Division,Forest Department Sarawak,Kuching,Sarawak93250,Malaysia5Graduate School of Global Environmental Studies,Kyoto University,Yoshida-Honmachi,Sakyo,Kyoto606-8501,Japan
*Corresponding author, E-mail: kawashibi@gmail.com
- Zoological Research的其它文章
- Description of two new species of Hemiphyllodactylus(Reptilia: Gekkonidae) from karst landscapes in Yunnan, China, highlights complex conservation needs
- Functional explanation of extreme hatching asynchrony: Male Manipulation Hypothesis
- Metagenomic analysis reveals presence of different animal viruses in commercial fetal bovine serum and trypsin
- Phylogenetic analysis of combined mitochondrial genome and 32 nuclear genes provides key insights into molecular systematics and historical biogeography of Asian warty newts of the genus Paramesotriton(Caudata: Salamandridae)
- Dwindling in the mountains: Description of a critically endangered and microendemic Onychodactylus species (Amphibia, Hynobiidae) from the Korean Peninsula
- Whole-genome resequencing reveals molecular imprints of anthropogenic and natural selection in wild and domesticated sheep

