3,6-bis (2,2,2-trinitroethylnitramino)-1,2,4,5-tetrazine. Structure and energy abilities as a component of solid composite propellants
Anatoly G. Korepin, Natalia M. Glushakova, David B. Lempert, Anatoly I. Kazakov,Gennady V. Shilov, Denis V. Korchagin, Vadim M. Volokhov, Elena S. Amosova,Sergey M. Aldoshin
Institute of Problems of Chemical Physics, Ac. Semenov Avenue 1, Chernogolovka,142432, Russian Federation
Keywords:Nitrogen heterocycles Quantum chemistry Enthalpy of formation 3,6-bis(2,2,2-trinitroethylnitramino-1,2,4,5-tetrazine Crystal structure Energetic ability
ABSTRACT The work addresses to the study of the molecular and crystal structure and properties of a new energyintensive compound 3,6-bis(2,2,2-trinitroethylnitramino)-1,2,4,5-tetrazine(NBTAT),first obtained by the authors in 2020.NBTAT compound crystallizes in the monoclinic system,space group P2(1)/n,density at room temperature 1.939 g/cm3.The energies of crystal packing and pairwise intermolecular interactions in NBTAT and its unnitrated analogue BTAT were calculated, and their comparative analysis was carried out.The enthalpy of formation of NBTAT molecules was calculated by quantum-chemical methods using Gaussian 09,and the enthalpy of formation of NBTAT in the solid phase(618 kJ/mol)was estimated.The energy capabilities of NBTAT as an oxidizer of solid composite propellants are estimated.It is shown that in metal-free compositions NBTAT is significantly superior to ammonium perchlorate (AP), dinitramide ammonium salt (ADN), HMX, BTAT at all stages of rocket systems, and is comparable to the superdense CL-20 yielding to the latter at the lower stages and slightly winning at the upper stages.
1. Introduction
In the last two or three decades,research on the possibilities of synthesizing new high-energy compounds that can increase the ballistic capabilities of mixed solid rocket propellants and other energy compositions has been developing very actively. Much attention is paid to compounds based on high-enthalpy N-heterocycles substituted by various fragments with oxidizing properties(nitro groups,nitramine, etc.) [1-6].
The combination of a high-enthalpy high-nitrogen N-heterocycle(with one or more rings) and several nitro groups in a single molecule allows one to provide high values of the enthalpy of formation (ΔH) along with a high oxygen saturation coefficient α(α = 2O/(4C + H)), thereby allowing such compounds to become effective oxidizers for high-energy compositions. One of the basic frameworks for creating high-enthalpy compounds is tetrazine.There are many energy-intensive compounds created on one or more tetrazine cycles [7-15].
Recently [16], the preparation of another representative of the class of nitrosubstituted 1,2,4,5-tetrazine, namely 3,6-bis(2,2,2-trinitroethylnitramino)-1,2,4,5-tetrazine (NBTAT, CHNO;α = 1.14), has been described (Fig.1).
This compound was obtained by nitration of 3,6-bis(2,2,2,-trinitroethylamino)-1,2,4,5-tetrazine (BTAT, Fig. 2) described in Ref. [15]with a mixture of nitric acid and trifluoroacetic anhydride[16].
In this paper, the ballistic capabilities of NBTAT as a component(oxidizer)of solid composite propellants(SCP) are investigated. For this purpose, a quantum-chemical study of the structure and thermochemical properties of NBTATand BTAT in the gas phase and X-ray diffraction analysis of crystalline NBTAT were performed,and NBTAT sublimation heat and enthalpy of formation ΔH(s)in the solid state were estimated.The study of the ballistic capabilities of NBTAT was carried out in comparison with well-known and widely used components: octogen (HMX), ammonium perchlorate (AP), ammonium dinitroamide(ADN)and hexanitrohexaazaisowurtzitane(CL-20).
2. Experimental section
2.1. NBTAT synthesis

Fig.1. NBTAT
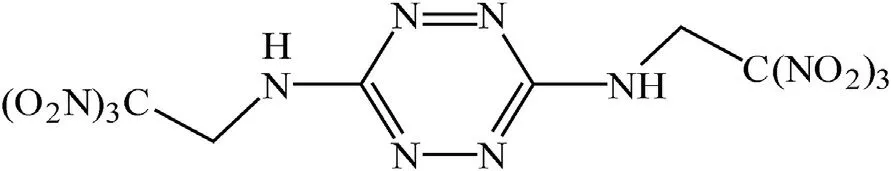
Fig. 2. BTAT.
NBTAT was synthesized by nitration of BTAT in two stages[16];in the first one BTAT was nitrated with 99-100% nitric acid, in the second one the nitration was continued in the same medium, but with the addition of trifluoroacetic acid anhydride,as described in Ref. [16]. The yield was 32%. The nature of the substance obtained was confirmed by NMR and IR spectra,the structure of the product was confirmed by X-ray structural analysis.
2.2. Methods of quantum-chemical research
The quantum-chemical calculation of the enthalpy of formation in the gaseous phase(ΔH(g))was performed within the Gaussian 09 package[17]using the combined CBS-4M method and using the hybrid density functional B3LYP [18,19] with the basis set 6-311+G(2d,p), which had already proven itself in molecular calculations.
The AIMAll program package[20]was used to analyze the wave function by QTAIM (Quantum Theory of Atoms in Molecules)methods.Energies of intermolecular contacts were calculated from Espinosa-Lecomte formula E ≈1/2ν(r),where ν(r)is the potential energy density at the bond critical point [21].
2.3. Calculation of the NBTAT formation enthalpy in the solid phase

where Tis the melting point or the temperature of the beginning of decomposition,K [22].
2.4. X-ray study of NBTAT
The X-ray diffraction experiment was performed on a CCD diffractometer Agilent XCalibur with EOS detector (Agilent Technologies UK Ltd,Yarnton,Oxfordshire,England)at 300 K.Collection and processing of the data, determination and refinement of the unit cell parameters were performed within the CrysAlis PRO program [23].
The structure was solved by the direct method. Positions and temperature parameters of non-hydrogen atoms were refined in isotropic and then in anisotropic approximation by the full matrix least-squares method (LS).
The hydrogen atoms positions were calculated geometrically and refined with the riding model.
The X-ray crystal structure data have been deposited with the Cambridge Crystallographic Data Center, with reference codes CCDC 2083269 (300K)-2083270(100K).
Table 1 presents main crystallographic data of NBTAT.
2.5. Method for estimating the energy and ballistic characteristics of SCPs based on the NBTAT oxidizer
Calculations of the specific impulse Isp and temperature in the combustion chamber Tc (at pressures in the chamber and at the nozzle exit of 4.0 and 0.1 MPa,respectively)were performed using the TERRA code for calculating high-temperature chemical equilibria [24]. The analysis of the effectiveness of the investigated components was carried out according to the algorithm described in our previous works [25,26].
To compare the ballistic efficiency of compositions having different densities when used in engines with different volumemass characteristics, the so-called values of effective impulses Ief(n) at different stages of rocket systems were used (n is the stage number) [27].
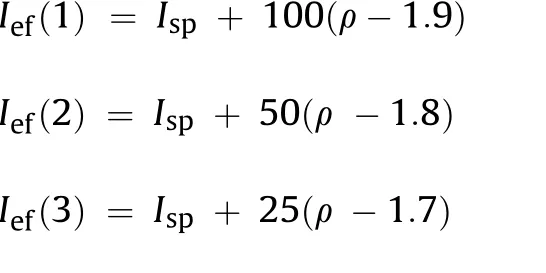
where ρ is density of the propellant, g/cm
These values characterize the ballistic efficiency of the propellant at the corresponding stages of the rocket systems.
As applied to new components, their efficiency at the upper stage is usually of greatest interest, where the propellant mass is 4-10 times lower than at the lower one, since the cost of the components and their sensitivity are extremely important. However, in this article, we analyze the effectiveness of NBTAT at all three stages,comparing with the effectiveness of such well-known oxidants as ammonium perchlorate (AP), ammonium dinitramide(ADN), and hexanitrohexaazaisowurtzitane (CL-20). Propellant compositions that do not contain metal are considered.
To ensure satisfactory physical and mechanical characteristics of SCPs and the rheological properties of uncured propellant mass,the compositions must contain sufficient amount of polymer binder.This is usually achieved when the volumetric content of the binder is not lower than 18-19 vol.%. Thus, special attention was paid to this value, in all formulations it is not lower than 18 vol.%. For correct comparison, all SCP formulations considered in this work have approximately the same volume fraction of the binder,18.0 ± 0.1 vol.%. The calculation methodology was presented in more detail in our previous works [25,28,29].
3. Results and discussion
3.1. Quantum-chemical research
Results of quantum-chemical calculations have shown that NBTAT can exist in the gas phase as two isomers (Fig. 3a and b).
Structure of NBTAT trans-isomer is shown in detail in Fig. 4.
As follows from Figs.3b and 4 for the trans-isomer,the structure of the molecule practically does not differ from that of BTAT(Fig.5)except for the >NH groups being replaced by the >NNOgroups.
The complete information on the bond lengths and angles can be found in the Supplementary data.
Cis and trans isomers have very close values of the enthalpy of formation in the gas phase (ΔH(g)) (622 and 618 kJ/mol, accordingly). This is significantly higher than the value for BTAT(ΔH(g) = 422.0 kJ/mol) [15], which is structurally similar to NBTAT. Though in most cases the replacement of hydrogen atomsby the nitro group(i.e.the transition from R-NH-Rto R-N(NO)-R) leads to a decrease in the enthalpy of formation (per mole),NBTAT, the nitrated derivative of BTAT, is characterized by the increased value of the enthalpy of formation, which is due to a significant stress in the NBTAT molecule because of the substitution of two hydrogen atoms in BTAT by the nitro groups.
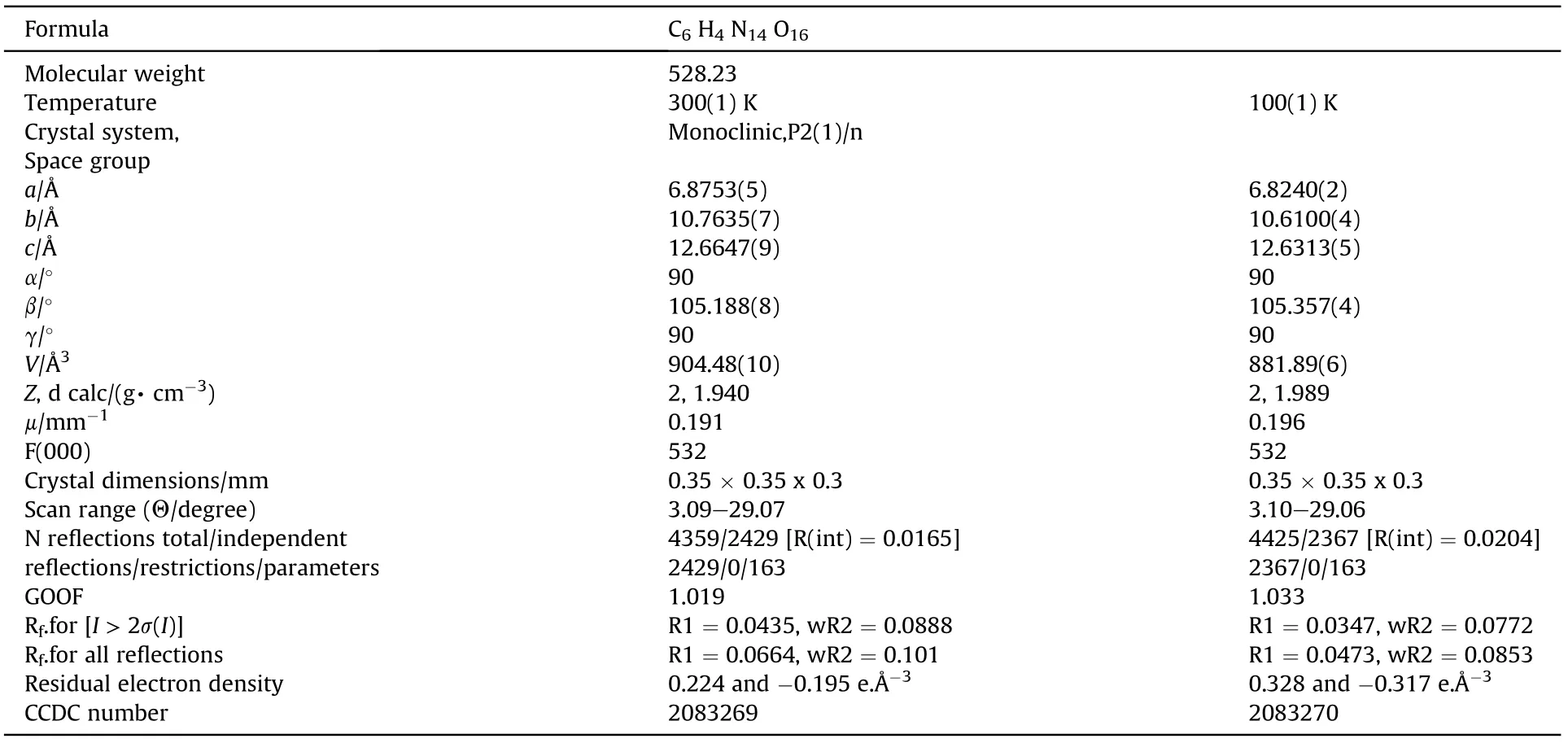
Table 1 Main crystallographic data of NBTAT at 300 and 100 K.
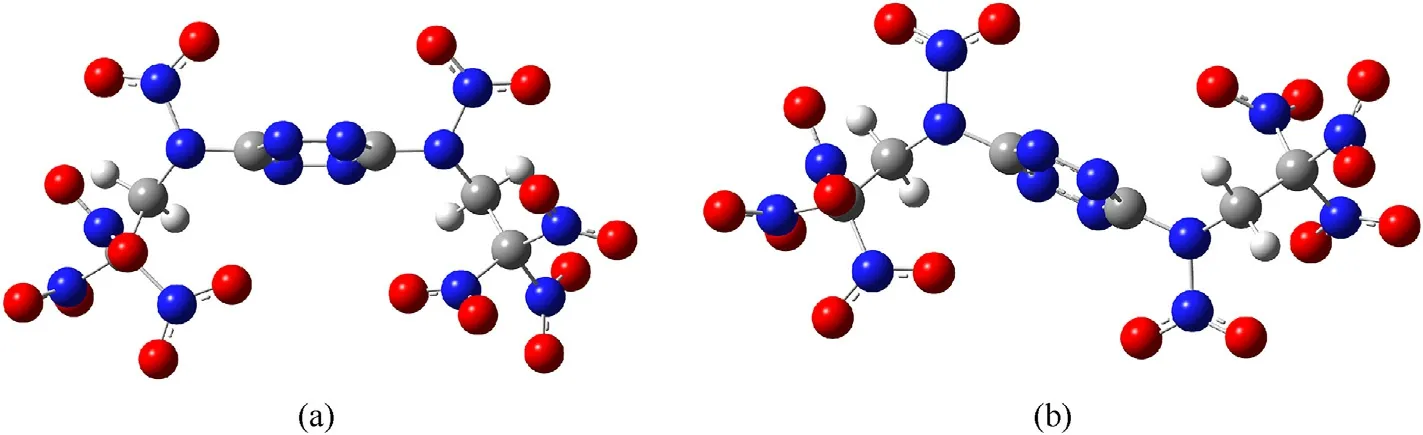
Fig. 3. Molecular structures of NBTAT in the gas phase, (a) - cis-isomer, (b) - trans-isomer.
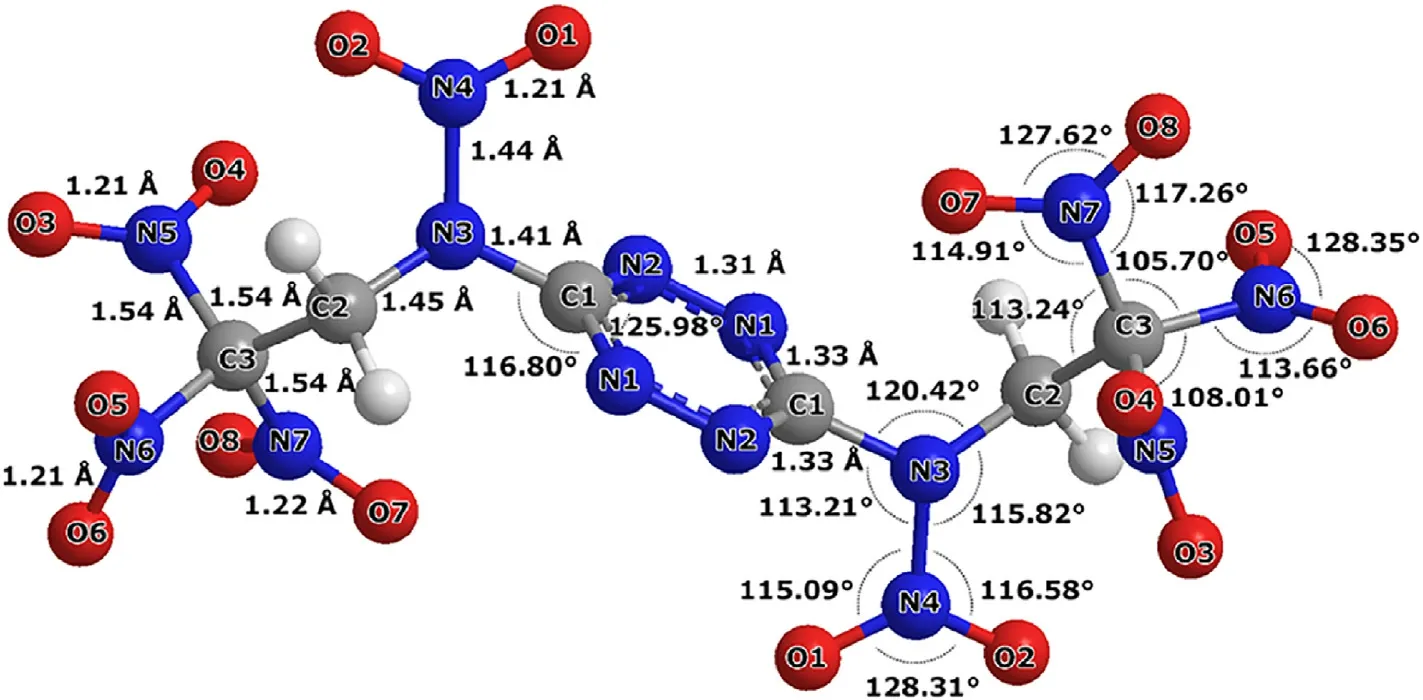
Fig. 4. Structure of NTBAT trans-isomer in the gas phase.
3.2. Estimation of the formation enthalpy NBTAT in solid state
The calculation of the formation enthalpy in the solid phase ΔH(s) for the NBTAT component is based on the value of its ΔH(g) (618 kJ/mol) in the gas phase, as described in the Experimental Section.Table 2 summarizes the formation enthalpy values of NBTAT and BTAT in the solid phase.
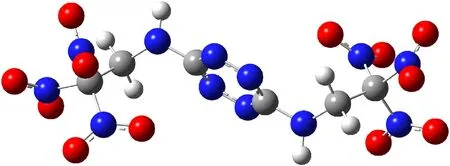
Fig. 5. Molecular structure of BTAT in the gas phase.
The increased sublimation enthalpy of BTAT in comparison with that of NBTAT may be related to the possibility of forming hydrogen bonds of the hydrogen atoms of the NH groups with the oxygen atoms of the nitro groups of neighboring molecules in the BTAT crystal. Substitution of the hydrogen atom in BTAT by the nitro group increases ΔH(s) of the resulting NBTAT by 105 kJ/mol for each hydrogen atom substituted by the nitro group.
Since the thermochemistry of triazine derivatives is more advanced than the thermochemistry of tetrazine ones,it is useful to determine the difference between the values of ΔH(s) of disubstituted triazine and tetrazine with the same substituents, which allows ΔH(s)of tetrazine derivatives,even not yet synthesized,to be estimated based on ΔH(s)of known triazine-based analogues.
In order to estimate ΔH(s)of compounds containing tetrazine cycles based on the values of ΔH(s) of structurally similar compounds containing triazine cycles, the energy increment of the substitution of the triazine cycle by the tetrazine cycle should be calculated. For this purpose, ΔH(s) of N,N-dinitro-N,N-bis(2,2,2-trinitroethyl)-1,3,5-triazine-2,4-diamine (BTNNT, Fig. 6)structurally similar to NBTAT has been calculated.
The calculation is based on the literature data on ΔHof triazine(171.7 kJ/mol) [30], 2,4,6-triazido-1,3,5-triazine (1053 kJ/mol) [31],and 6-azido-N,N-dinitro-N,N-bis(2,2,2-trinitroethyl)-1,3,5-triazine-2,4-diamine (BTNNAT, Fig. 7) (630.1 kJ/mol) [32]. The value of ΔH(s) for BTNNT was found to be 336.3 kJ/mol. Comparison of this value with ΔH(s)of NBTAT(548 kJ/mol)shows that the difference between the values of ΔH(s) of disubstituted triazine and tetrazine with the same substituents is approximately equal to 210 kJ/mol, which can be used to estimate ΔH(s) of tetrazine-series compounds based on the known values of ΔH(s)of triazine-series compounds with the same substituents.
3.3. Crystal structure of NBTAT
From X-ray analysis data, NBTAT crystallizes in the monoclinic system;the crystal structure was refined in the centrosymmetrical space group P2(1)/n.The molecular structure is presented in Fig.8.The molecule is centrosymmetrical, with the asymmetric part being half a molecule. The bond lengths and angles are presented in Supporting Information.
Fig. 6. BTNNT.
Fig. 7. BTNNAT.
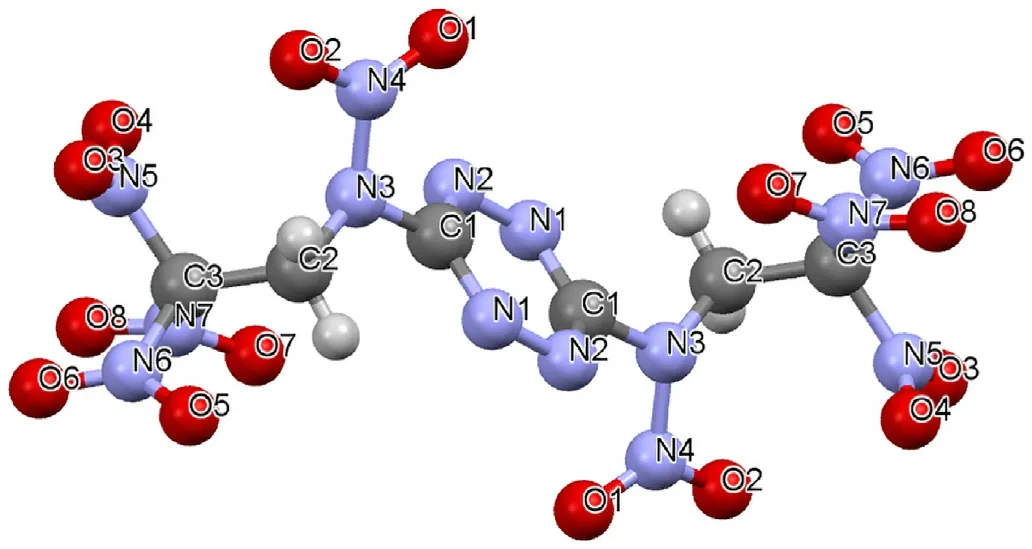
Fig. 8. Molecular structure of NBTAT from X-ray analysis.
Bond lengths and angles in NBTAT and BTAT molecules do not show any significant distinctions from the values typical for compounds containing trinitroethyl groups (such as bis(2,2,2-trinitroethyl)amine (BTNA), N1-(2,2,2-trinitroethyl)-1H-tetrazole-1,5-diamine (TTD), and N1,N5-bis(2,2,2-trinitroethyl)-1Htetrazole-1,5-diamine (BTTD)). Geometrical parameters of the tetrazine rings are compatible both for the compounds under consideration, and with the values observed for 3,6-diamino-1,2,3,5-tetrazine. The main distinction between NBTAT and BTAT molecules is observed for the rotation angles of the substituents at the tetrazine rings,which is due to the replacement of the hydrogen atoms of the amine groups by the nitro groups.This induces steric repulsion between the lone pairs of the oxygen atoms of the nitro group (O(1)) and the nitrogen atom of the tetrazine ring (N(2)),which appears as a considerable increase in similar torsion angles N1C1N5C5 (N3C2N6C3) ~10.6(average for BTAT) and N1C1N3C2~27.6(for NBTAT).
In addition,for more thorough comprehension and prediction of the properties, it is important to compare the crystal structures of NBTAT and the initial BTAT, the latter previously presented by Klap¨otke and G¨obel[15].BTAT crystal structure is characterized bythe presence of infinite polymeric chains formed due to the hydrogen bonds between the molecules.These chains form a threedimensional structure through van der Waals interactions. Fig. 9 shows the molecular structure of this compound obtained from the X-ray analysis. It should be noted that the replacement of the hydrogen atoms at N5 and N9 nitrogen atoms of the amine groups by the NOgroups brings to a considerable increase in the product density from 1.869 g/cm(for BTAT)to 1.998 g/cm(calculated from X-ray data at 100 K).BTAT crystallizes in the orthorhombic system;volume of the unit cell is 1557.7(2)?,the number of formula units Z = 4, molecular weight is 438.23. NBTAT crystallizes in the monoclinic system, V = 878.01(9) ?, Z = 2, molecular weight is 528.23.

Table 2 The values of the formation enthalpy of NBTAT and BTAT.
The volumes corresponding to two formula units are 778.9 ?and 878.0 ?for BTAT and its nitrated derivative, respectively, i.e.,BTAT has a denser packing than its derivative. Thus, the density growth is due to the molecular weight increase. The volume corresponding to the equal number of formula units increased by 13%,while the molecular weight increased by 21%.
Fig. S1 in the Supplementary data shows the crystal packing of BTAT. Fig. S1 (a) shows the fragment of BTAT crystal packing. The neighboring polymeric bands (Fig. S1 (b)) are stretched at some angles to each other, their planes are not parallel, and the angles change by about one degree with the temperature increase.Fig.10b shows intermolecular hydrogen bonds (IHB) of N-H…N type and their values at 100 K.
Fig. S1 (c) presents the projection of the fragment of NBTAT crystal packing on bc plane. As distinct from BTAT for which intermolecular hydrogen bonds are determining in the crystal formation, the crystal structure of its nitrated derivative is stabilized mainly due to van der Waals interactions with the NOgroup participation.
For a more detailed comparison of the crystal structures of NBTAT and its initial non-nitrated analog,the energies of the crystal packing and individual pairwise interactions have been calculated.
As follows from the comparative analysis of pairwise interactions in the crystal structures of BTATand NBTAT,the hydrogen atoms substitution by the nitro groups results in the decrease in the energy of the crystal lattice from-228.4 to-189.5 kJ/mol.Despite multiple van der Waals contacts with participation of the oxygen atoms of the nitro groups in the NBTAT structure, the presence of the intermolecular hydrogen bonds in the structure of the initial BTAT, which are the determining ones, contribute to the essential stabilization of the crystal packing. Indeed, the energy of N-H…N contacts exceeds the energy of all other non-bonded interactions almost twice, while the total energy of such pairwise interaction(Fig. S2 in the Supplementary data) is also twice as high as that of pairwise interactions in other directions (-80.8 vs -35.6 kJ/mol,Fig. S3 in the Supplementary data).
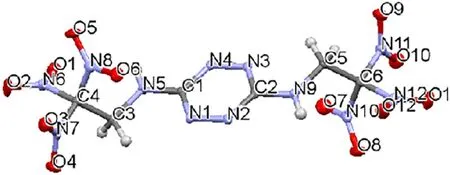
Fig. 9. Molecular structure of BTAT from X-ray analysis [15].

Fig.10. Temperature dependence of the unit cell volume.
3.4. Temperature X-ray investigations of NBTAT and BTAT
To understand thermal transformations in the compounds under consideration, temperature investigations of the unit cell parameter changes were performed in the temperature range of 100-370 K. Fig. 10 presents the temperature dependence of the unit cell volume.There are three regions in the diagram which can be approximated by straight lines. With the temperature increase,the lines slope in these regions increases,for example,in the range of 100-170 K the slope is 0.146 ?/K,and in the range of 350-370 K it is 0.497 ?/K. The variation of the unit cell parameters is known to be due to anharmonicity. Thus, anharmonistic effects in the NBTAT crystal grow with the temperature increase. In the temperature range of 100-350 K the crystal keeps well upon cooling,heating and repeated cooling.However in the range of 350-370 K the crystal decomposes mechanically upon heating. Even if we managed to keep the crystal upon heating at low speed,the crystal still decomposed mechanically either during the parameters refinement,or X-ray diffraction experiment.Some chips had black inclusions, which could be the result of micro explosions. Such mechanical destruction can be explained by a sharp change in the crystal volume upon heating above 350 K, which is confirmed by the experimental data, i.e., in two first temperature ranges the volume growth is 0.48 and 1.46 ?per each 10 K,while in the third range such growth is 4.97 ?, which is about 0.5% of the unit cell volume.In low-temperature ranges the percentage is about 3 and 6 times less, respectively. Upon heating, the volume of the external layers of the crystal increases quickly, while the inner part of the crystal is not hot yet,which leads to arising the stress in the crystal and, hence, its destruction.
X-ray experiments were performed in each of the three mentioned temperature ranges,and crystal structures were refined using the atoms coordinates obtained at 100 K. This was possible because no polymorphic transformations were observed in the whole temperature range, and the symmetry was the same.Table S4 in the Supplementary data represents the torsion angles calculated at different temperatures.
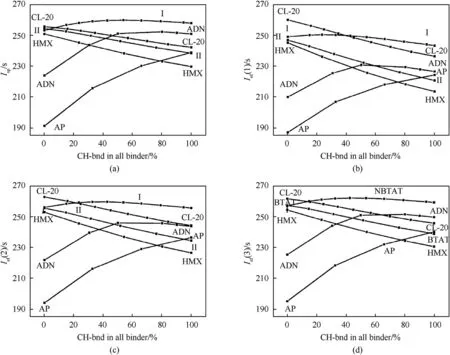
Fig.11. Values of Isp(a),Ief(1)(b);Ief(2)(c);Ief(3)(d)for metal-free compositions with 18 vol.%of the binder(CH-bnd and Act-bnd mixture)as dependent on the basic solid filler and the CH-bnd mass fraction in the whole binder.
We can see that changes in the molecule induced by the temperature are mainly in the range of 2. However, there are some torsion angles which vary by 4-5upon heating from 100 to 360 K(O5-N6-C3-N5,O5-N6-C3-C2,O5-N6-C3-N7,O7-N7-C3-C2).It can be noted that such changes occur in the largest trinitromethyl fragment of the molecule,which has the maximum degrees of freedom. Fig. S4 in the Supplementary data shows molecular structures from three temperature regions; the atoms are presented as ellipsoids of 50%probability.At 360 K the amplitudes of thermal vibrations increase for the whole molecule,not only for the terminal groups that are characterized by the presence of strong librational vibrations.It is worth attention that in the central part of the molecule the amplitudes of N1,N2 nitrogen atoms in the ring are somewhat larger than those of C1 carbon atom and N3 nitrogen atom,which provides the assumption of small librational vibrations of this central fragment around the C1-N3 direction.As follows from the comparison of mean-square deviations of these atoms at various temperatures, such qualitative conclusion can be applied to the whole temperature range.
Thus,not only the outer part of the molecule,but also its central part,undergo librational vibrations.Table S5 in the Supplementary data presents shortened intermolecular contacts at 100 K and 360 K. As expected, with the temperature increase their number reduces considerably,because at temperatures above 350 K a sharp growth of the unit cell volume is observed.
Quantum-chemical calculations of the molecule energy dependence on the values of internal rotation angles showed that rotations of the trinitromethyl fragment around the C-C bond, of the nitro groups in the trinitromethyl fragment around the C-N bond, and of the whole substituent around the C-N bond have the lowest energetic barriers(less than~14.6 kJ/mol),while for the rotation of 2,2,2-trinitroethyl group around the same C-N bond the barrier is~42.0 kJ/mol(Fig.S5 in the Supplementary data).We have earlier observed the similar behavior for tris(2,2,2-trinitroethylnitroamine)triazine molecule [[33]]. For the compound under consideration, some decrease of the barrier values is observed, which could be due to the reduction of the steric influence of bulky substituents in the para-position as distinct from three bulky substituents in the meta-position.
The temperature X-ray studies have been also performed for the initial BTAT crystal in the temperature range of 100-335 K. The crystal structure of BTAT keeps in the whole temperature range.With the temperature increase, intermolecular hydrogen bonds N-H…N considerably weaken because the N…N distance increases by 0.07-0.08 ?; the number of shortened contacts between the polymeric bands decreases, and mean-square deviations of the atoms increase by approximately thrice. The crystal kept mechanically stable in the studied temperature range.
3.5. Energy characteristics of NBTAT-based SCP
Comparison of the energy and ballistic characteristics of NBTAT,ammonium dinitramide HNO- ADN, HMX, and CL-20 was carried out by comparing the Isp and Ief(n)values achieved in metalfree formulations, i.e. in binder + oxidizer binary systems. The volume content of the binder in all compositions, as indicated in the Experimental Section,was 18±0.1%.Two known binders were chosen as the basis for the mixed binder: the active (Act-bnd -CHNO;ΔH=-757 kJ/kg;ρ=1.49 g/cm)[34]and the hydrocarbon one (CH-bnd - CHO;ΔH= -393 kJ/kg;ρ=0.92 g/cm)[34].When using oxidants with different values of α, the most optimal from the energy point of view is a binder with the α value lying in a certain range depending on the α value of the oxidizing agent,i.e.,oxidizers with a high value of α(above~1)require mainly a hydrocarbon binder,for α of the oxidizer below 0.8 an active one is required, for 0.9 <α < 1.2, the mixture binder CHbnd+Act-bnd with a certain mass ratio of Act-bnd and CH-bnd is optimal [35].
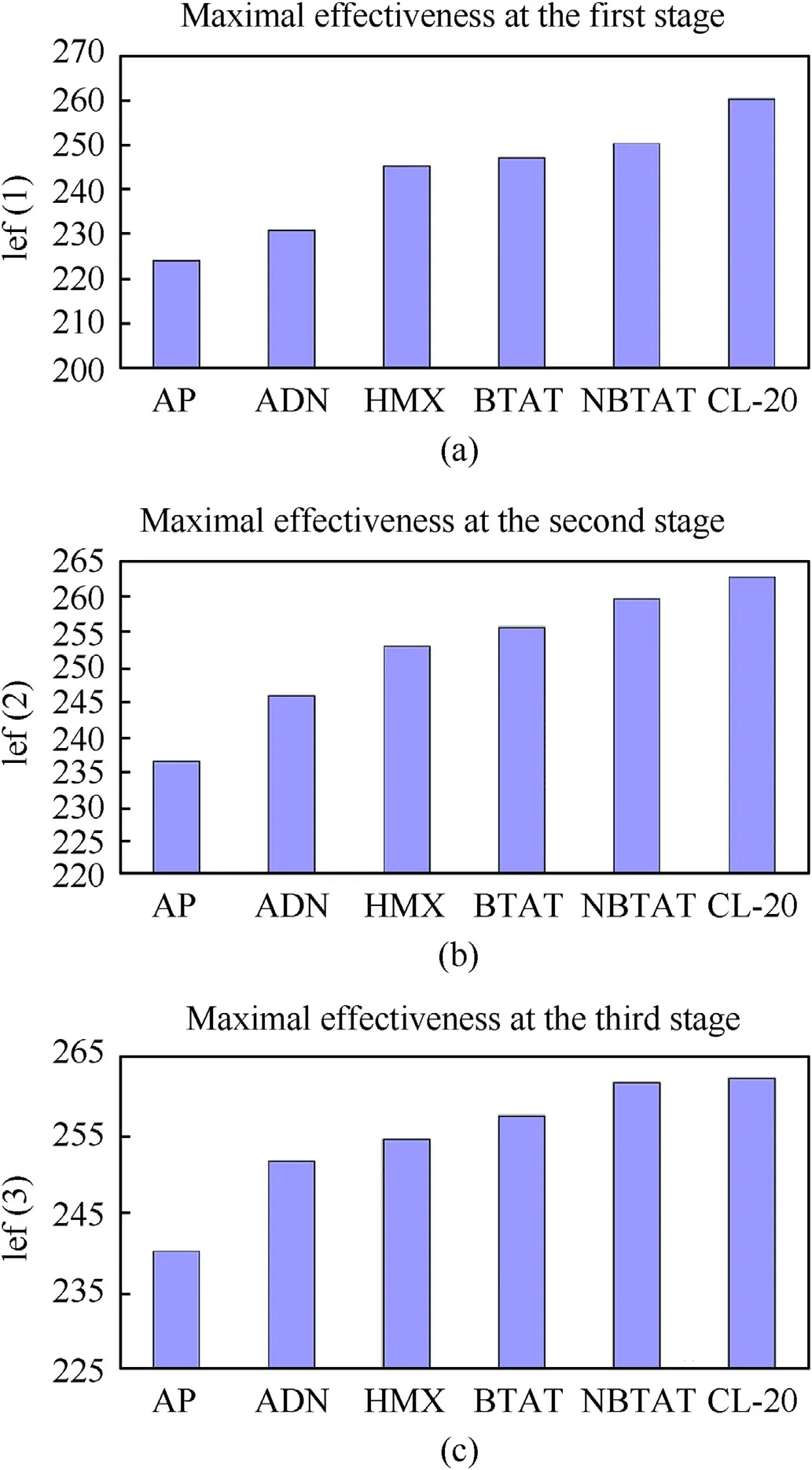
Fig.12. Maximum achievable values of Ief(1)(a);Ief(2)(b)and Ief(3)(c)of metal-free compositions with 18 vol.% of the binder (mixture of CH-bnd with Act-bnd) while using various basic solid fillers.
Fig.11 shows the calculated data of the Isp and Ief(n)values for metal-free compositions containing 18 vol% of the mixed CHbnd+Act-bnd binder and an oxidizer(NBTAT,AP,ADN,HMX or CL-20) as dependent on the CH-bnd mass fraction in the mixture of CH-bnd+Act-bnd binder.As expected,the Isp and Ief(n)values in compositions based on oxidants with high α values (AP, α = 2.25 and ADN,α = 2.0) increase with an increase in the fraction of CHbnd in the mixed binder (CH-bnd + Act-bnd), while for oxidants with low α values(HMX,α=0.667,BTAT and CL-20,α=0.8),they decrease. For NBTAT, the maximum values of Ief (n) are clearly observed for CH-bnd/(CH-bnd + Act-bnd) ratio being equal to 0.4-0.5.In terms of ballistic efficiency at stage 1,CL-20 is the most effective, judging by the value of Ief (1); with the optimal formulation of the binder,it is ahead of NBTAT by about 10 s,which is very significant.Note that BTAT, which has the same elemental content as CL-20,loses to CL-20 in any formulations,since both the density and ΔHof BTAT are lower than those of CL-20.In terms of Ief(1),the optimized BTAT formulations lose to CL-20 by about 12 s.Under the same conditions(binder content of 18 vol.%),formulations with NBTAT outperform formulations with BTAT, HMX, ADN and AP by 3.5;5.0;20,and 26 s,respectively.In terms of ballistic efficiency at stage 2, judging by the value of Ief (2), CL-20 is still the most effective;with an optimal binder formulation,it is ahead of NBTAT by about 3 s. But in comparison with other components under consideration,NBTAT wins under the same conditions:in terms of Ief (2), the compositions with NBTAT outperform those with BTAT,HMX, ADN and AP by 4.0; 5.7; 13.8, and 23.2 s, respectively. In terms of ballistic efficiency at stage 3,judging by the value of Ief(3),NBTAT outperforms even CL-20,albeit quite a bit(about 0.5 s).The advantage of NBTAT over BTAT, HMX, ADN and AP is significant,approximately 4.8; 7.8; 10.6, and 22 s, respectively.
The effect of using each of the studied components on the ballistic efficiency of metal-free compositions with 18 vol.% of binder at different stages of rocket systems is shown in Fig.12, so we can confidently consider NBTAT as one of the most energy-intensive SCP-oxidizers, which is, in terms of ballistic efficiency, ahead of AP, ADN, and HMX, and approximately equivalent to CL-20.
Since the calculated value of the explosive detonation velocity is one of the important indicators of the energetic material, we have calculated it for NBTAT to get detonation velocity of 9.37 km/s at a detonation pressure of 41.6 GPa.
Research into the performance of NBTAT is ongoing. As for the level of thermal stability,the fact that NBTAT melts at about 110С(with decomposition)does not yet indicate its poor stability.ADN,one of the most powerful oxidizing agents of SCPs, melts at about 85C,but thermal stability of ADN-based compositions in the range of manufacture and operation (20-60C) is quite satisfactory for using ADN as a main filler of SCPs [36].
As for the sensitivity to mechanical stress, such investigations will be performed in near future.So far,it is only possible to give a preliminary estimation of the impact sensitivity by comparing the maximum heats of explosive transformation Q[37],where Qis total heat release upon conversion of all H atoms into water,provided that all the remaining O atoms are used for COformation.NBTAT has Q= 6590 kJ/kg, while Qcorresponds to 6695;5858; 6940 5515; 6987, and 6799 for HMX; TNT; CL-20 (hexanitrohexaazaisowurtzitane);dinitroguanidine,benzotrifuroxane,and RDX, respectively. Thus, we can tentatively consider the impact sensitivity of NBTAT to be close to that of HMX.
4. Conclusions
(1) Parameters of the NBTAT molecule in the gas phase have been determined by quantum-chemical methods (CBS-4M within Gaussian 09). There are two isomers (cis and trans),with the enthalpy of formation of the trans isomer being 4 kJ/mol lower than that of the cis isomer. These values of enthalpies of formation are significantly higher than those of BTAT, from which NBTAT was obtained by nitration.
(2) Main crystallographic data for NBTAT have been determined by X-ray method. Its X-ray density at room temperature is 1.939 g/cm. The geometry of NBTAT in the gas and solid phases is essentially similar. The absence of intermolecular hydrogen bonds because of the replacement of the hydrogen atoms at the amine nitrogen atoms by the nitro groups causes a relative decrease in the crystal structure energy of NBTAT as compared to the initial BTAT, which is responsible for a somewhat less thermal stability of NBTAT.
(3) Based on the calculated formation enthalpy of NBTAT in the gas phase and its sublimation enthalpy calculated according to the Trouton's rule, the value of the formation enthalpy of NBTAT in the solid phase is estimated equal to 546 kJ/mol.
(4) It has been shown that NBTAT, by its energy properties, can perform as one of the most powerful SCP-oxidants. In compositions without metal and metal hydrides, it significantly surpasses ammonium perchlorate, ammonium dinitramide,HMX,BTAT,and is at the level of CL-20,yielding to the latter at the lower stages and slightly winning at the upper ones.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research was supported by The Ministry of Science and Higher Education of the Russian Federation (Agreement with Zelinsky Institute of Organic Chemistry RAS No 075-15-2020-803).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.06.002.
- Defence Technology的其它文章
- A new model for the expansion tube considering the stress coupling:Theory, experiments and simulations
- Experimental and analytical assessment of the hypervelocity impact damage of GLAss fiber REinforced aluminum
- Effect of the microporous structure of ammonium perchlorate on thermal behaviour and combustion characteristics
- Modeling of bistatic scattering from an underwater non-penetrable target using a Kirchhoff approximation method
- Rapid preparation of size-tunable nano-TATB by microfluidics
- Mechanical behavior of Ti-6Al-4V lattice-walled tubes under uniaxial compression

