Photocurrent Response Properties of 2,6?Bis(3′?pyridyl)?tetrathiafulvalene Based Zn/Co Coordination Polymer
LI Jun?LiWEN Yi?HangWANG Le?JiaLI XingXIAO Xun?Wen
(1School of Material Science and Chemical Engineering,Ningbo University,Ningbo,Zhejiang 315211,China)
(2School of Material and Chemical Engineering,Ningbo University of Technology,Ningbo,Zhejiang 315211,China)
(3College of Chemistry and Life Science,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
Abstract:This is the first time that coordination polymers(CPs)with the ligand 2,6?bis(3′?pyridyl)?tetrathiafulva?lene(3′?py?TTF?3?py)has been reported.These two CPs are isostructural with formulated as[Zn(3′?py?TTF?3?py)2(TPA)]n(1)and[Co(3′?py?TTF?3?py)2(TPA)]n(2),(H2TPA=terephthalic acid).The X?ray single?crystal diffraction shows that 1 and 2 are 2D coordination polymers.The photoelectric experiment results show that the S…S interac?tions and the center metal in the CPs have an important impact on their photocurrent responses.CCDC:2054408,1;2054409,2.
Keywords:coordination polymers;tetrathiafulvalene;S…S interaction;photocurrent responsive
Coordination polymers(CPs)are metal?organic hybrid materials that are formed with metal ions or met?al?based clusters and organic ligands.Due to the vari?ous structures and intriguing properties of CPs,such materials are one of the research hotspots in recent years[1?7].Moreover,many research groups paid enor?mous attention to multifunctional CPs which showed combination properties[8?9].In recent years,the CPs with redox activity property have made great achievements,and it has shown applications prospects in the fields of porous conductive materials,electrochromic material,sensors,energy conversion,and storage materials[10?13].It is a promising method to introduce the redox?active organic ligands to construct redox?active CPs.Tetrathi?afulvalene(TTF)and its derivatives,as well?known redox?active organic molecules,are widely used as donor molecules from organic conductors or semicon?ductor compounds.The incorporation of TTF units into CPs was reported recently,and the results showed that it provided an effective strategy to get redox?active CPs which had potential value in optoelectronic devices[14?17].
Bipyridine,especially 4,4′?bipyridine(4,4′?bpy)as an organic ligand was often used to construct CPs,and the Yaghi group reported the first CuI?4,4?bpy net?work as metal?organic frameworks(MOFs)in 1995[18].Dai group reported a redox?active analogue of 4,4′?bpy,2,6?bis(4′?pyridyl)?tetrathiafulvalene and get adianet?work with redox?active properties[19].And our group paid much attention to the TTF derivatives,especially the TTF derivative containing pyridine group due to the various inorganic?organic hybrid compounds formed by the N?M coordinative bond[20].Moreover,pre?viously reported results make it clear that substituent groups on the TTFs unit affected the stacking pattern of TTFs markedly,which is one of the key factors affect?ing the properties of TTF derivatives[21?22].In this work,an analogue of 2,6?bis(4′?pyridyl)?tetrathiafulvalene,2,6?bis(3′?pyridyl)?tetrathiafulvalene(3′?py?TTF?3?py,Fig.1)(only with the different position of N atom on the pyridine unit)was selected to prepare CPs with the redox?active property.Based on our knowledge,CPs based on 3′?py?TTF?3?py have not been reported.It is a new CP system in which new intriguing phenomena would be expected to be discovered.As expected,two redox?active coordination polymers with the formulas[M(3′?py?TTF?3?py)2(TPA)]n(M=Zn(1),Co(2))were successfully prepared by using terephthalic acid(H2TPA)as a co?ligand.Herein the synthesis,crystal structures,and photocurrent responsive properties of the two CPs are described.
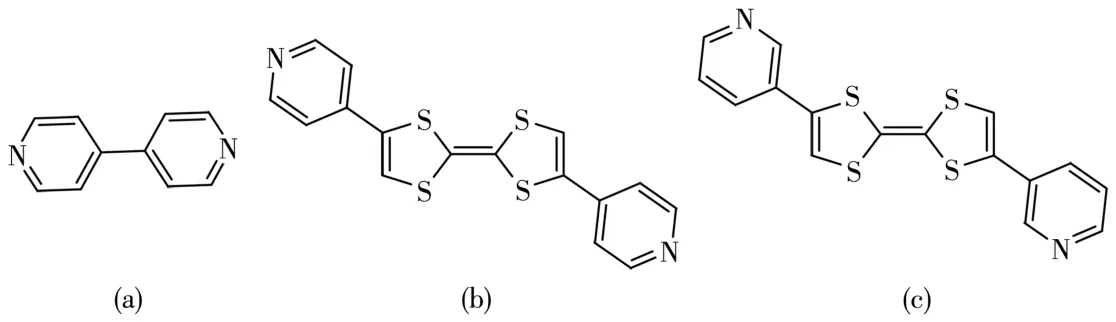
Fig.1 Molecular structures of(a)4,4′?bpy,(b)2,6?bis(4′?pyridyl)?tetrathiafulvalene,and(c)3′?py?TTF?3?py
1 Experimental
1.1 Materials and measurements
All reagents and solvents of reagent grade were obtained from commercial channels and used directly without further purification.The TTF derivative(3′?py?TTF?3?py)was synthesized by referring to the previous?ly reported method[23].The powder X?ray diffraction(PXRD)data of complexes 1 and 2 were collected by a Bruker D8 focus X?ray diffractometer(CuKαradiation,2θ=5°?50°,λ=0.154 18 nm,40 kV,40 mA)at room temperature.CHI660E electrochemical workstation was used to carry out the photocurrent response experi?ments.
1.2 Preparation of complexes 1 and 2
3′?py?TTF?3?py(0.035 8 g,0.1 mmol),H2TPA(0.051 g,0.3 mmol),and Zn(NO3)2·6H2O(0.104 g,0.3 mmol)were combined in a stainless?steel grinding jar with six grinding balls,then one drop of DMF was added and the material was ground at 30 revolutions per sec?ond with a Retsch M400 ball milling machine.Com?plex 1 was obtained after one hour.Yield:88.03 mg,31% based on Zn(Ⅱ).Anal.Calcd.for C40H24N4O4S8Zn(%):C,50.76;H,2.56;N,5.92.Found(%):C,50.74;H,2.54;N,5.94.
The synthesis of complex 2 was the same as that of complex 1,except that Co(NO3)2·6H2O(0.029 g,0.1 mmol)was used instead of Zn(NO3)2·6H2O.Yield:32.9 mg,35% based on Zn(Ⅱ).Anal.Calcd.for C40H24N4O4S8Co(%):C,51.11;H,2.57;N,5.96.Found(%):C,51.14;H,2.56;N,5.98.
1.3 Structure determination
At room temperature,two single crystals with sizes of 0.18 mm×0.26 mm×0.3 mm(1)and 0.14 mm×0.2 mm×0.26 mm(2)were collected on Bruker APEX?ⅡCCD diffractometer with MoKαradiation(λ=0.071 073 nm).The software of Crys Alis Pro Agilent Technologies was used to analyze the frames of data,index the reflec?tions,and determine the lattice constants,absorption correction,and data reduction.Using Olex2,this struc?ture was solved by the ShelXT structure solver using the inherent phase method,and refined by the full?matrix least square method using the SHELXL onF2.The hydrogen atoms were assigned anisotropic dis?placement factors and were included in the final refin?ing cycles by using geometric constrain,and all non?hydrogen atoms were anisotropically refined.The details of all pertinent crystallographic data and struc?ture refinements for complexes 1 and 2 are summarized in Table 1.The selected bond lengths and angles for 1 and 2 are listed in Table 2.
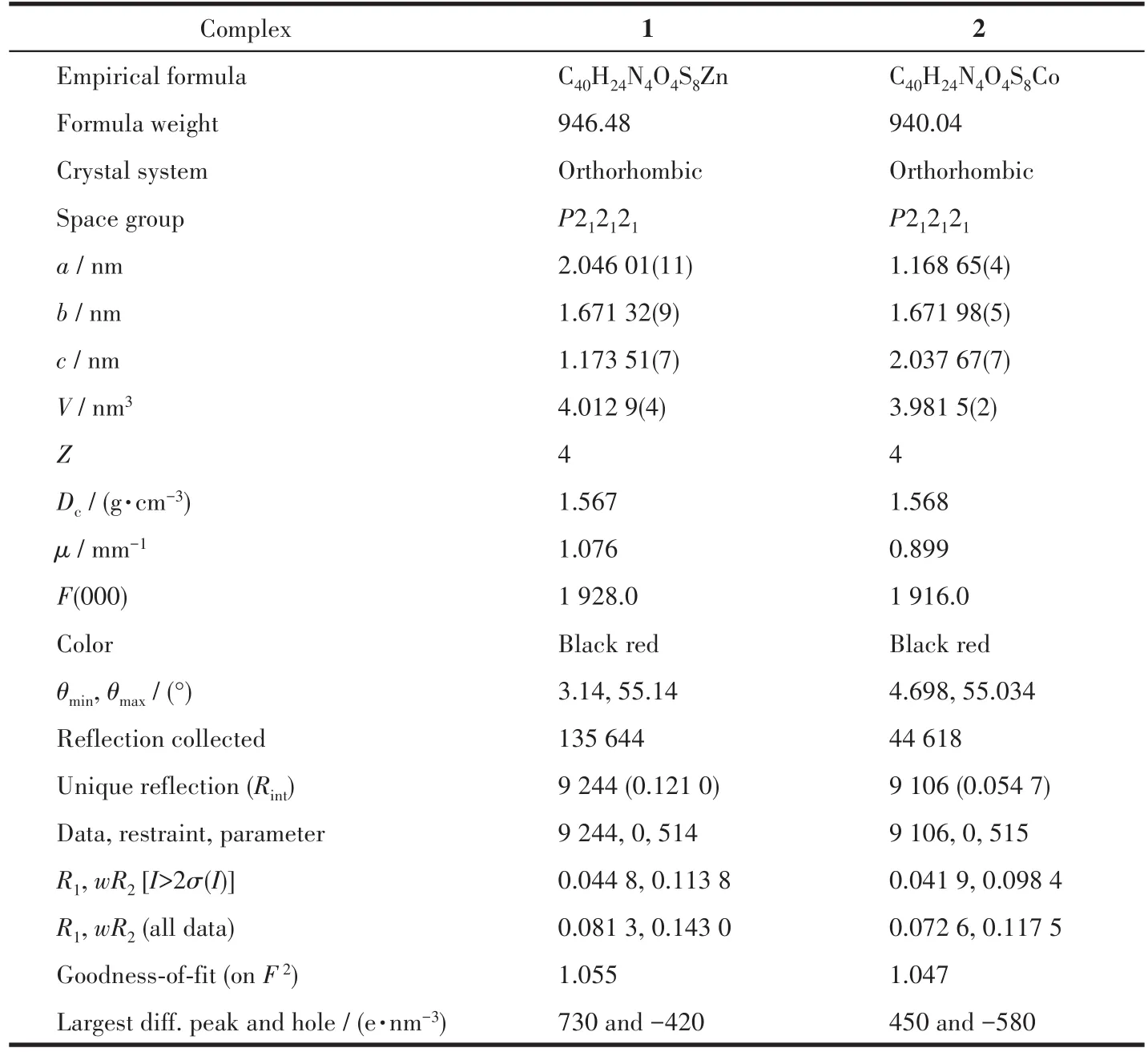
Table 1 Crystal data and structure refinement for complexes 1 and 2
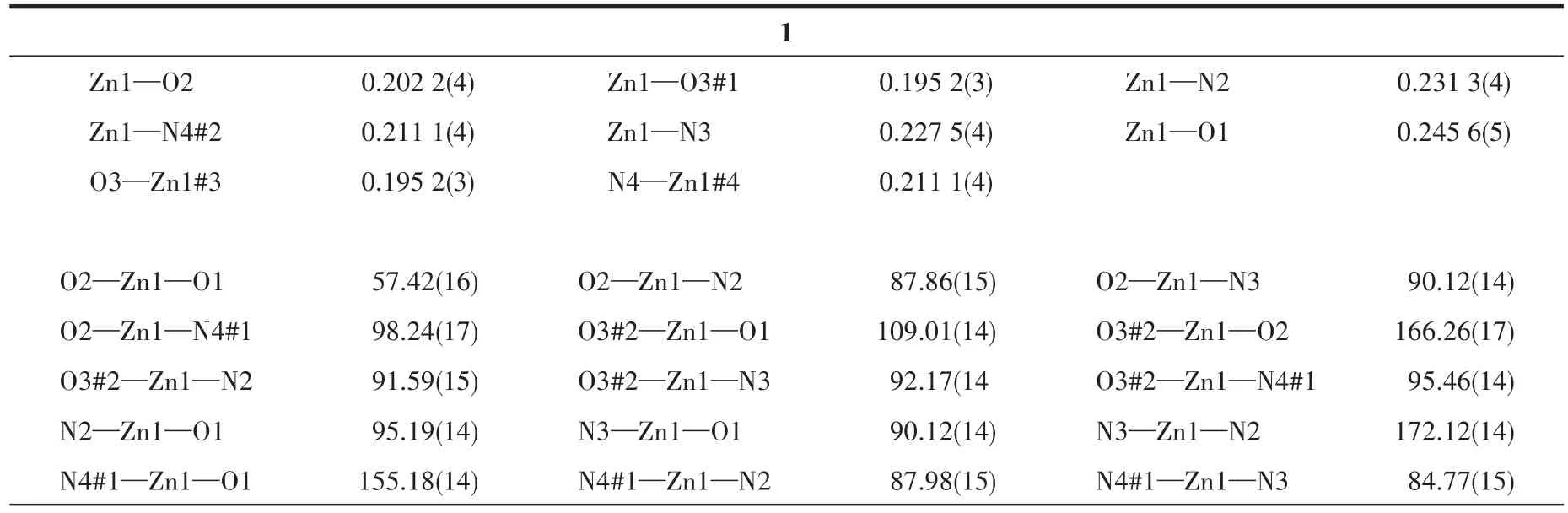
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
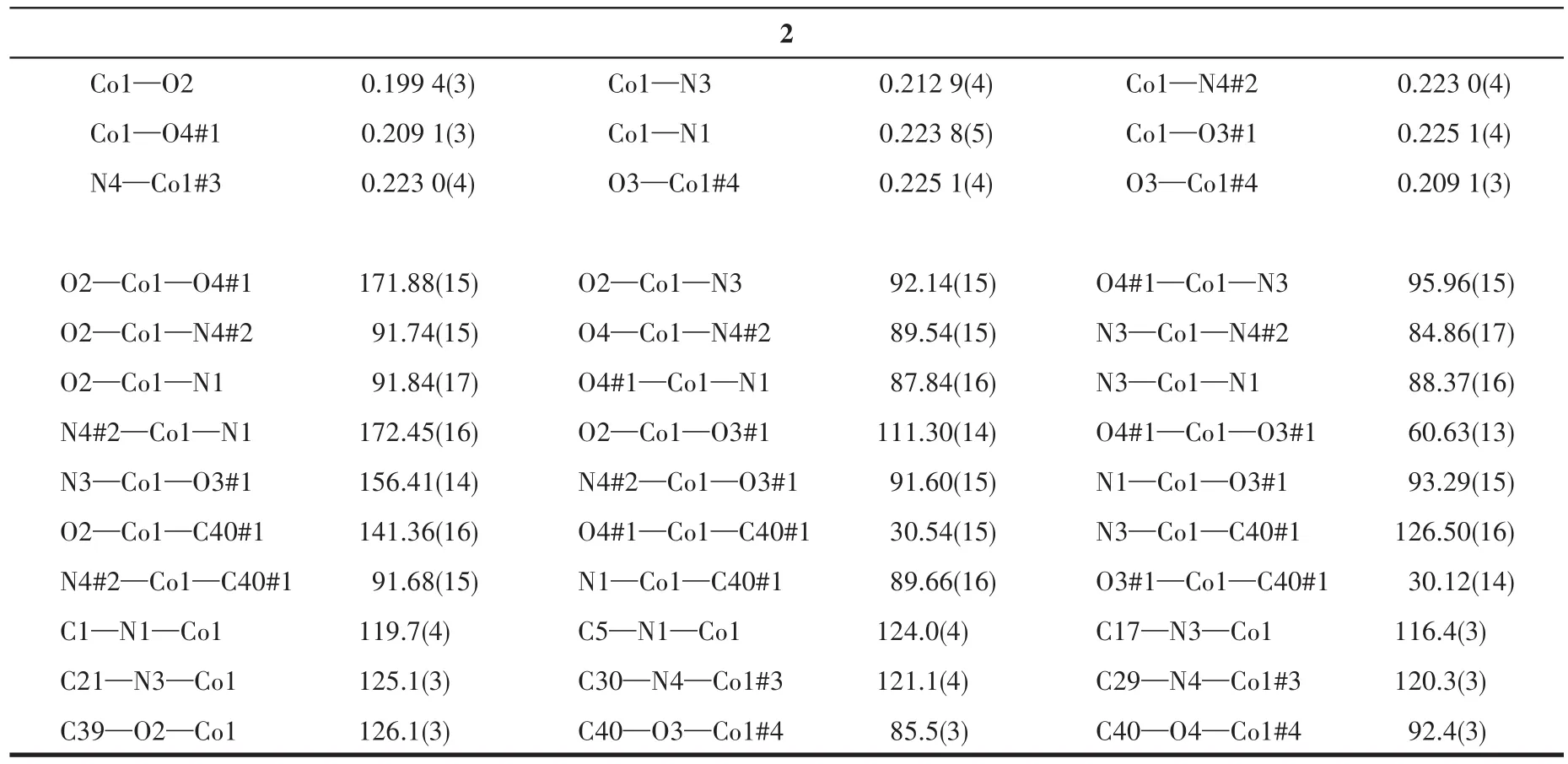
Continued Table 2
CCDC:2054408,1;2054409,2.
2 Results and discussion
2.1 Description of the crystal structures
The crystal structures of 1 and 2 are shown in Fig.2 and 3,respectively.Since the two complexes are isostructural,we only describe the structure of zinc complex 1 in detail.Single?crystal X ?ray structural analysis shows that complex 1 crystallizes in orthogo?nal space groupP212121.The asymmetric unit,as shown in Fig.2,is composed of one crystallographically independent zinc ion,two 3′?py?TTF?3?py molecules,and one TPA2?anion.The Zn(Ⅱ) ion is six?coordinate by three oxygen atoms(O1,O2,O3)from TPA2?ligand,and three nitrogen atoms(N2,N3,N4)from two 3′?py?TTF?3?py ligands to form a distorted octahedron coordination geometry(Fig.2a).The Zn—O lengths are 0.202 2(4),0.202 2(4),and 0.195 2(3)nm,and the Zn1—N2,Zn1—N3,and Zn1—N4#2 lengths are 0.231 3(4),0.227 5(4),and 0.211 1(4)nm,respectively.

Fig.2 (a)Coordination environments of Zn(Ⅱ)in complex 1;(b)1D structure of complex 1;(c)2D view of complex 1
The adjacent Zn(Ⅱ)ions are bridged by the TPA2?anion to form an infinite wavy 1D chain along thea?axis(Fig.2b).And the 1D chain is linked by 3′?py?TTF?3?py ligand to generate a 2D coordination framework which exhibits a ladder structure viewed along theb?axis(Fig.2c).
As shown in Fig.3,short S…S interactions(S3…S8 0.349 7 nm,S7…S6 0.355 7 nm)are observed with the di?coordinated 3′?py?TTF?3?py to the adjacent mono?coordinated 3′?py?TTF?3?py alonga?axis,which may facilitate efficient pathways for charge migration.
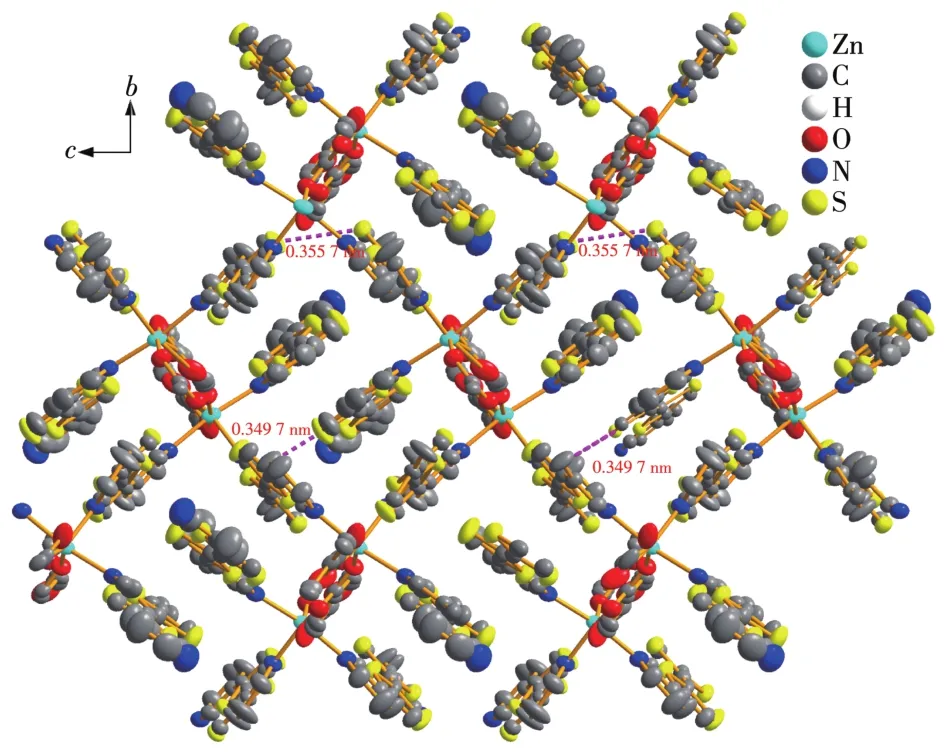
Fig.3 S…S interactions of 1 viewed along a?axis
2.2 PXRD analysis
The PXRD analysis for complexes 1 and 2 was performed to check their purity.As shown in Fig.4a and 4b.The main peaks in these simulated and experi?mental patterns were fitted well,which may illustrate the purity phase of these two crystals.
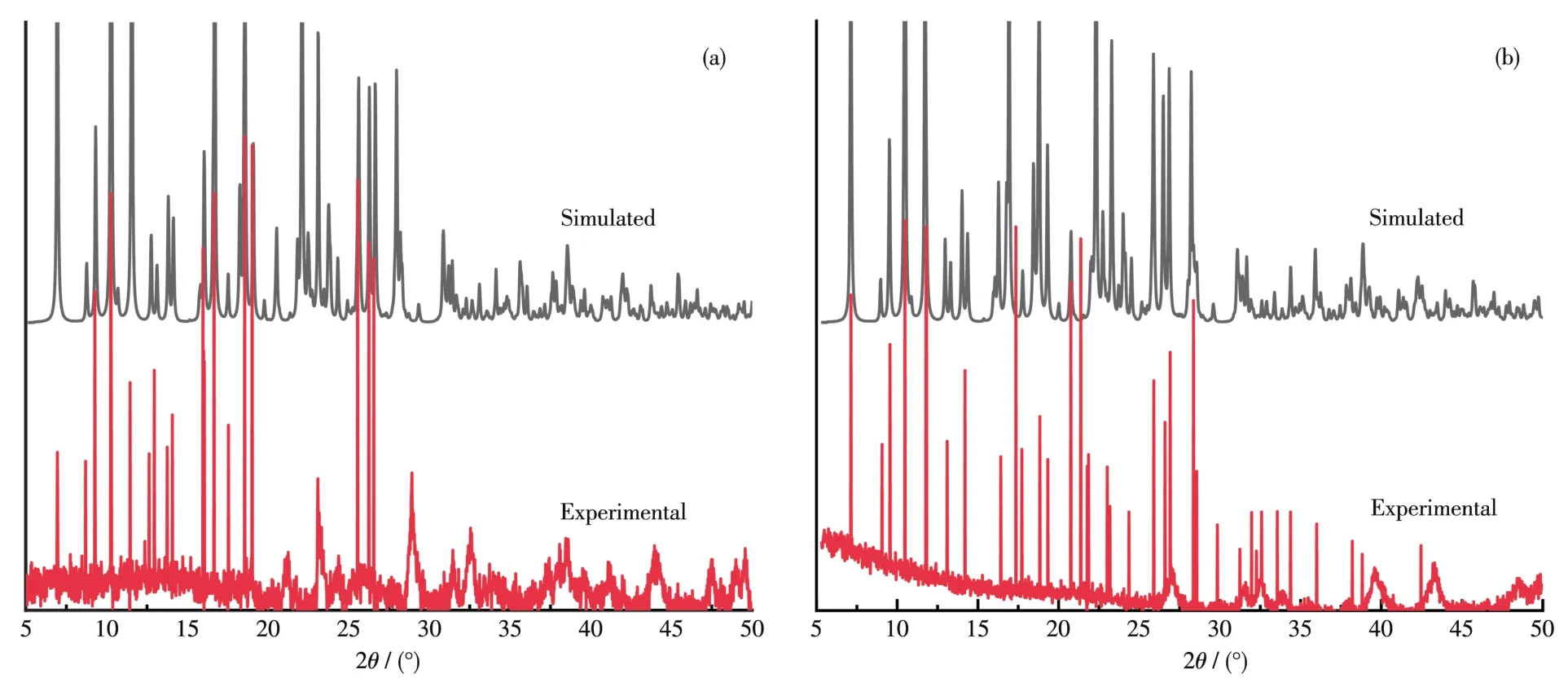
Fig.4 XRD patterns for experimental and simulated results of complexes 1(a)and 2(b)
2.3 Photocurrent response properties
To investigate the possibility of electron transi?tions,the molecular energy levels of complexes 1,2,and the ligand 3′?py?TTF?3?py were calculated using the Gaussian 09 program package.Fig.5 shows the HOMO and LUMO energies.The molecular orbital analyses of the 3′?py?TTF?3?py ligand show that the HOMO is mainly located on the TTF moiety,while the LUMO population almost shift to the pyridyl group with small contributions of the TTF moiety.For 1,the HOMO is almost located on one of the TTF moieties,while the LUMO is located on one of the pyridine groups and half of the TTF moieties.And for 2,the HOMO is almost located on one of the TTF moieties,while the LUMO shifts to the coordination center Co?pyridine and half of the TTF moiety.And the HOMO?LUMO energy gap of 2 is 1.710 0 eV,narrower than that of 1(2.907 4 eV)and 3′?py?TTF?3?py(3.317 3 eV).Therefore,the transition is also attributed to intra?molecular charge transfer from the TTF moiety to the pyridine,and the charge transfer is enhanced by metal coordination.
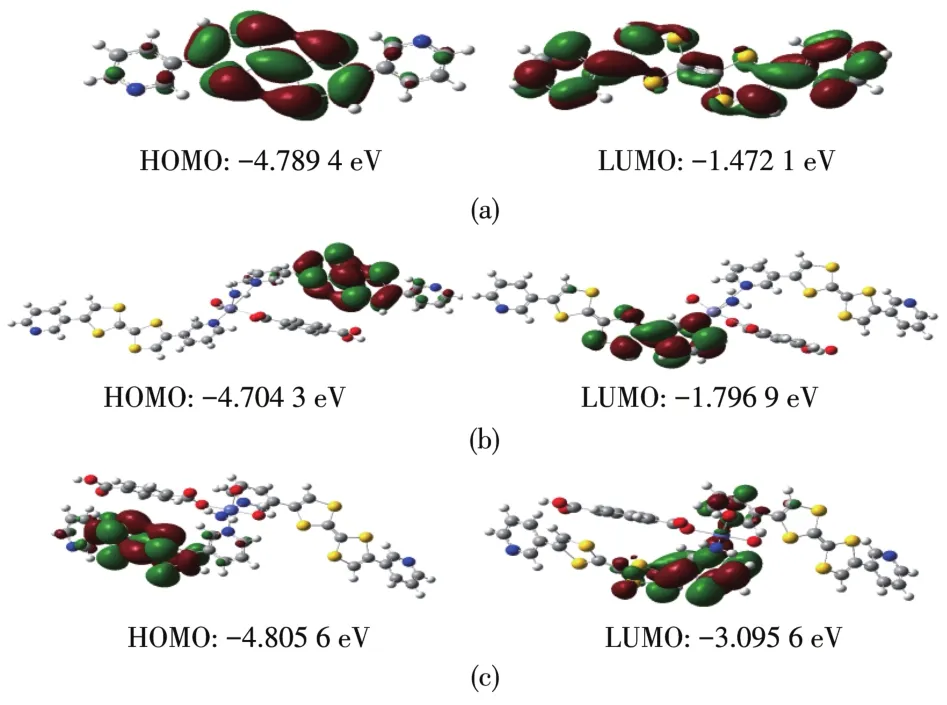
Fig.5 HOMO and LUMO of 3′?py?TTF?3?py(a),1(b),and 2(c)
Charge?transfer compounds of TTF derivatives usually exhibit photoelectroactivity[24?25].These are the first reported CPs based on 3′?py?TTF?3?py,so,it is interesting to study the photocurrent response property of such kind of CP.The photocurrent measurement of CPs 1,2 together with 3′?py?TTF?3?py ligand was per?formed and shown in Fig.6.Once irradiated with Xe light,the photocurrent was instantly obtained at the highest value.On the other hand,the photocurrent can be immediately reduced to its initial level when the Xe light was off.The phenomenon can repeat several times.The photocurrent intensity of 1 and 2 was very stable without a decrease in the intensity which means that CPs are stable.More interesting,the current inten?sities of 2 were almost tenfold higher than that of 1 under the same experimental conditions,which indi?cates that the center metal has an important impact on the photocurrent responses.And the magnitude of pho?tocurrent density of CPs(1 and 2)was much higher than that of the original ligand.These results show that CPs with redox ligand are an effective strategy to get high photocurrent materials.
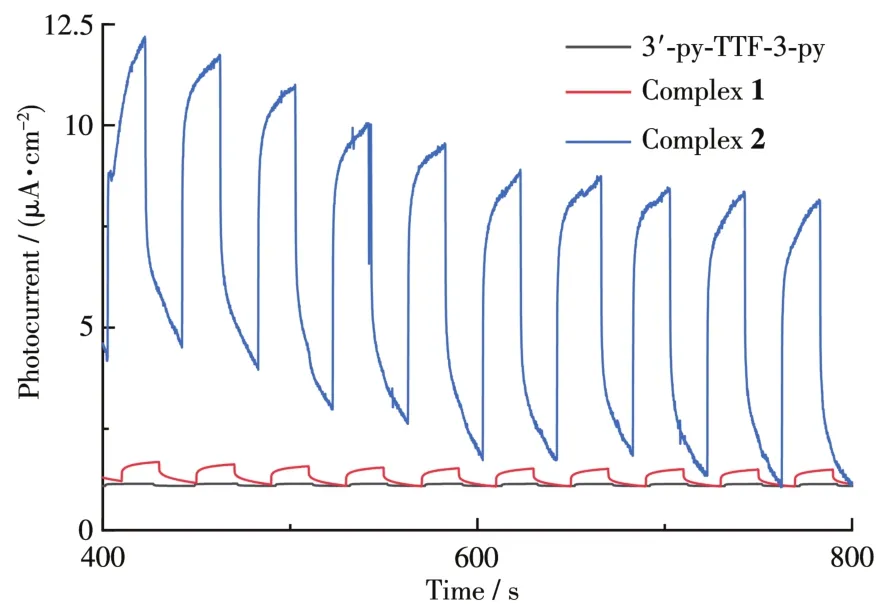
Fig.6 Photocurrent responses of 1 and 2
3 Conclusions
In conclusion,an extended analogue of 3,3′?bpy with a redox?active TTF moiety(3′?py?TTF?3?py)was selected to assemble CPs.To the best of our knowl?edge,this is the first time that CPs based on that ligand have been reported.The photoelectric active electrodes of the CPs were prepared,and the results suggest that the photocurrent intensity is related to the center metal of the CPs.More CPs with redox ligand is an effective strategy to get high photocurrent materials.
 無(wú)機(jī)化學(xué)學(xué)報(bào)2022年4期
無(wú)機(jī)化學(xué)學(xué)報(bào)2022年4期
- 無(wú)機(jī)化學(xué)學(xué)報(bào)的其它文章
- 《無(wú)機(jī)化學(xué)學(xué)報(bào)》投稿須知(NOTICE TO AUTHORS)
- N4O2?Donor Macrocyclic Schiff Base Ni(Ⅱ) Complex:Synthesis,Crystal Structure,DFT Study and Urease Inhibition Study
- Ag Nanoparticle?Doped Activated Microporous Carbon Spheres:An Efficient Adsorption/Catalysis Bifunctional Material for the Removal of Congo Red
- A Highly Sensitive and Multi?responsive Zn?MOF Fluorescent Sensor for Detection of Fe3+,2,4,6?Trinitrophenol,and Ornidazole
- Two 3D Microporous Zn?MOF for Fluorescence Sensing of Fe3+,Cr2O7 2-,and Acetone in Aqueous Solution
- Structures,Photoluminescent and Magnetic Properties of Three 2D Lanthanide Complexes
