Synthesis and Properties of Dinuclear Europium(III)Complex Containing 2-Benzoylbenzoic Acid①
YANG Ying-Qun LI Chang-HongLI Wei YANG Tian-Yi
a (Key Laboratory of Functional Metal-organic Compounds of Hunan Province & Key Laboratory of Functional Organometallic Materials of Hunan Province College, Hengyang 421008, China)
b (Department of Chemistry and Materials Science, Hengyang Normal University, Hengyang 421008, China)
c (School of Materials and Chemical Engineering, Hunan Institute of Technology, Hengyang 421002, China)
ABSTRACT A new europium(III) complex [Eu2(C14H9O3)6(2,2′-bipy)2]·(2,2′-bipy)2 has been synthesized with 2-benzoylbenzoic acid and 2,2′-bipyridine as ligands. Crystal data: triclinic, space group P1, with a = 13.5251(5), b =13.9453(5), c = 15.3582(5) ?, α = 76.922(3)o, β = 79.740(3)o, γ = 64.248(4)o, V = 2530.83(18) ?3, Dc = 1.496 g/cm3, Z =1, μ(MoKα) = 1.305 mm-1, F(000) = 1156, the final R = 0.0340 and wR = 0.0638. The Eu(III) ion is coordinated by eight atoms to give a distorted square antiprism coordination geometry. The complex exhibits antiferromagnetism in the temperature range of 300~2 K, and two intense fluorescence emission bands arising from the transitions of Eu3+: 5D0 →7F1 (595 nm) and 5D0 → 7F2 (618 nm), respectively. In addition, the fluorescence sensing properties of the complex in CrO42-solution were studied.
Keywords: europium(III) complex, crystal structure, magnetic and fluorescent properties;
1 INTRODUCTION
Rare earth elements have many excellent optical, electrical,magnetic and thermal properties. Owing to their very active chemical properties, they, together with many other elements,can form new materials with various uses and functions[1-5].Photoluminescence rare earth complexes can be used in such fields as fluorescent anti-counterfeiting materials, luminous coatings, agricultural films, luminous display, fluorescent probes and fluorescent immunoassay[6-10]. Of the 17 rare earth elements, europium and terbium are the two most used ones for the preparation of red and green materials due to their very good luminescence properties[11-13]. The luminescence mechanism of rare earth organic complexes is that the excited ligand transfers the excited energy to the central ion which then emits characteristic fluorescence. The luminescence intensity of rare earth complexes is affected by ligands. In order to synthesize high-efficient photoluminescence rare earth complexes, organic compounds with conjugated structure and good absorption of ultraviolet light are often selected as ligands. At the same time, the energy level difference between the triplet state of the selected ligands and the excited state of rare earth ions should be within a certain range. In addition,thermal vibration makes coordinated solvent molecules may consume some energies. Therefore, in the preparation of luminescent complexes, the introduction of appropriate auxiliary ligands can prevent solvent molecules from participating in coordination and enhance the luminescence performance of the complex[14].
Aromatic carboxylic acids have strong coordination ability with rare earth ions, and are often used to prepare rare earth complexes with high thermal stability and high efficiency photoluminescence properties[15,16]. 1,10-Phenanthroline,2,2′-bipyridine and their derivatives are often used as auxiliary ligands[17-21]. They replace the solvent molecules to coordinate with rare earth ions, which can not only reduce the non-radiation attenuation in the complex molecules, but also transfer more energy to rare earth ions. 2-Benzoylbenzoic acid is a good luminescent sensitizer of rare earth ions, and has been used in synthesizing rare earth complexes with good properties[22]. In this paper, we report a novel binuclear europium fluorescent complex prepared by self-assembly of europium ions with 2-benzoylbenzoic acid and 2,2′-bipyridine ligands. We have determined its structure, and studied its magnetic and fluorescence sensing properties.
2 EXPERIMENTAL
2. 1 Reagents and instruments
The reagents were commercially available and used without further purification. Elemental analyses were conducted with a PE-2400(II) apparatus. A crystalline sample was used for magnetic measurements in the range of 300~2 K on a MPMSSQUID magnetometer at a field of 2 kOe (1 kOe = 7.96 × 104A?m-1). Fluorescence spectra were recorded at room temperature on an F-7000 fluorescence spectrophotometer.
2. 2 Synthesis of the complex
A mixture of 0.58 mmol 2-benzoylbenzoic acid and 0.33 mmol europium(III) nitrate hexahydrate was dissolved in 10 mL water. The pH of the solution was adjusted to 5~6 by adding 1 M sodium carbonate. The resultant solution was poured into 20 mL glass test-tube. And then to this test tube were added 3 mL water-ethanol solvent with the volume ratio 1:2 and the mixed solvent of 0.94 mmol 2,2′-bipyridine and 4 mL ethanol. Afterwards, the test-tube was covered with plastic film. The mixture was kept at room temperature for slow diffusion. A month later, colorless single crystals appeared suitable for X-ray diffraction analysis. Yield: 34.8%. Anal.Calcd. (%) for (C124H86N8O18Eu2): C, 65.32; H, 3.80; N, 4.91.Found (%): C, 65.28; H, 3.79; N, 4.90.
2. 3 Structure determination and refinement
The X-ray diffraction measurement was performed at 293(2)K on a Bruker SMART APEX CCD area detector equipped with graphite-monochromatized MoKα(λ= 0.71073 ?) radiation. The structure was worked out by direct methods and refined by full-matrix least-squares with SHELXL-2015 program package[23]. Corrections forLpfactors, together with empirical adsorption adjustment, were applied and all non-H atoms were refined with anisotropic thermal parameters. The final refinement covering hydrogen atoms converged toR=0.0340 andwR= 0.0638 (w= 1/[σ2(Fo2) + (0.0208P)2], whereP= (Fo2+ 2Fc2)/3), (Δ/σ)max= 0.002 andS= 1.033.
3 RESULTS AND DISCUSSION
3. 1 Structural description
The molecular structure and coordination polyhedron for the central Eu(III) ion are shown in Fig. 1. Selected bond lengths and bond angles are given in Table 1.

Table 1. Selected Bond Lengths (?) and Bond Angles (°) of the Complex
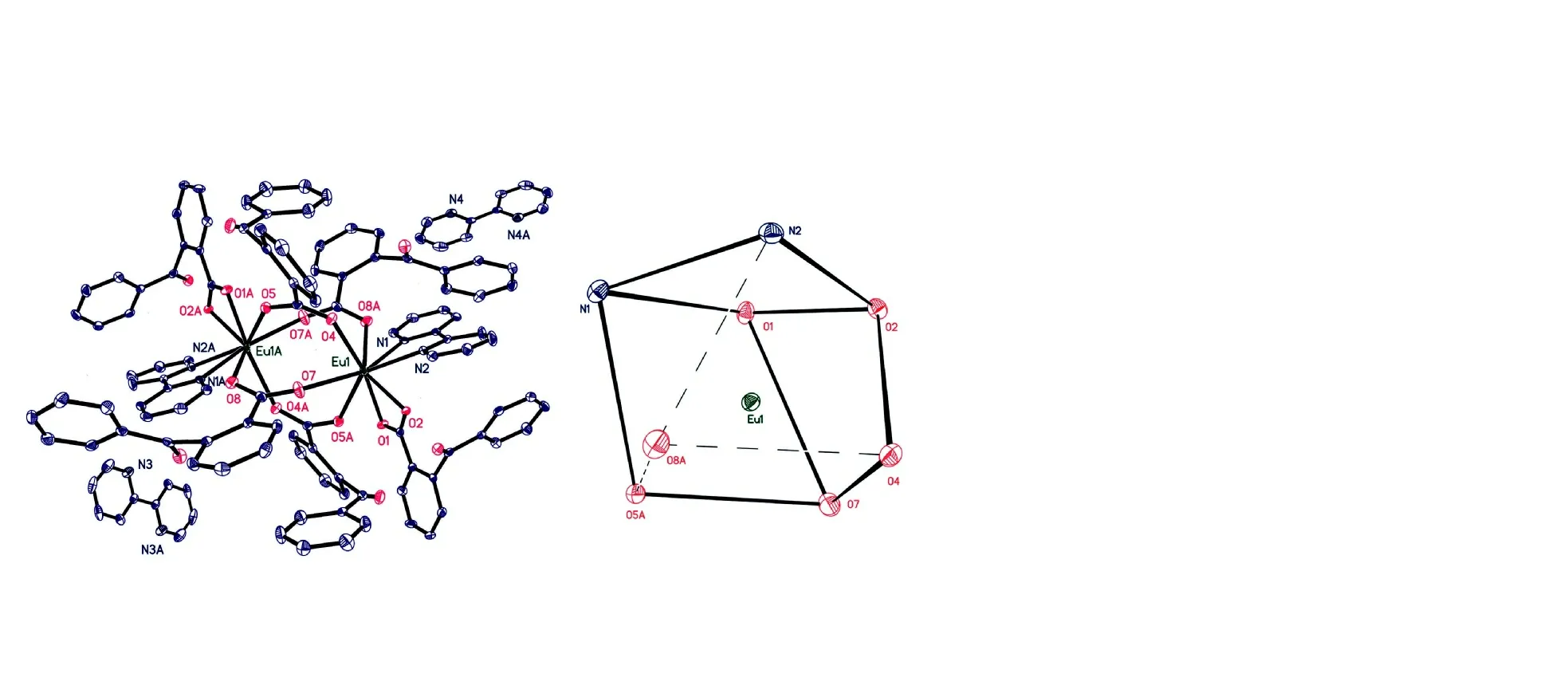
Fig. 1. Molecular structure of the complex and the coordination polyhedron for the Eu(III) ion at 30% displacement ellipsoids
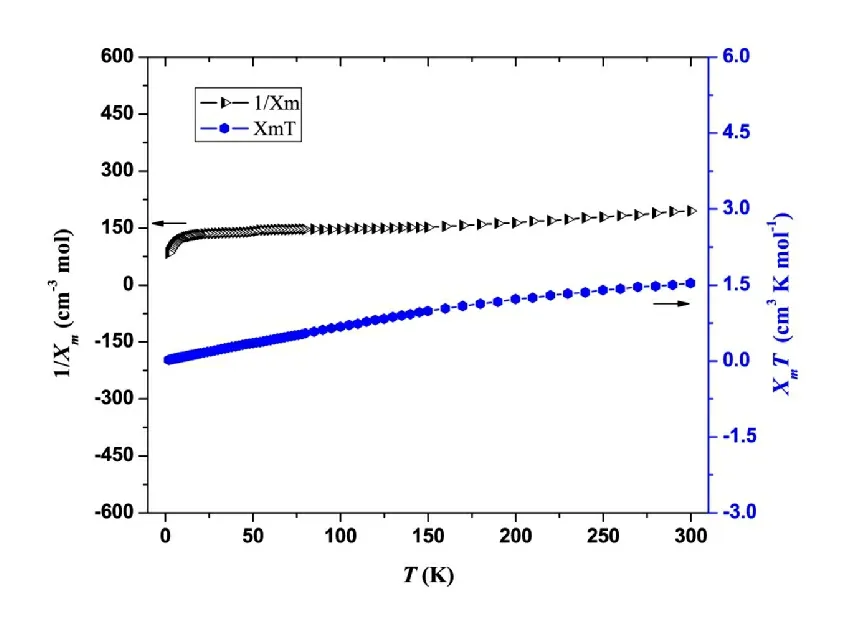
Fig. 2. Temperature dependence of the magnetic susceptibility of the complex in the form of XmT and 1/Xm vs. T
As illustrated in Fig. 1, the complex consists of two Eu(III)ions, six 2-benzoylbenzoic acid anions and four 2,2′-bipyridine molecules. Two Eu(III) ions are linked to four 2-benzoylbenzoic acid groups. The whole molecule displays a dinuclear structure. The distance between Eu(1) and Eu(1A) is 4.2743(5) ?. In the complex, 2-benzoylbenzoic acid anions coordinate with Eu(III) ions in two ways: bidentate chelating and bidentate bridging, respectively. Eu(III) ion is coordinated by six oxygen atoms from five 2-benzoylbenzoic acid anions,and by two nitrogen atoms from one 2,2′-bipyridine molecule.In the complex, there are two 2,2′-bipyridine molecules that do not coordinate with Eu(III) ion. The central Eu(III) ion adopts a distorted square antiprism coordination geometry(Fig. 1). In the coordination polyhedron (EuN2O6), O(1), O(2),N(2) and N(1) give the top plane of the square antiprism,while O(7), O(4), O(8a) and O(5a) determine the bottom plane. The dihedral angle of the two planes is 4.6o. The bond lengths between the oxygen atoms of 2-benzoylbenzoic acid and the central Eu(III) ion are between 2.3398(19) and 2.490(2) ?. These bond lengths are averaged to be 2.385 ?,which falls into the normal range[24]. Similarly, the bond lengths Eu(1)-N(1) (2.604(2) ?) and Eu(1)-N(2) (2.572(2) ?)are also normal. The N-Eu-O bond angles range from 69.44(8)to 147.46(8)o, and N(1)-Eu(1)-N(2) is 62.51(8)o, all of which are normal.
3. 2 Magnetic properties
The magnetic susceptibility was investigated with an applied magnetic field of 2 kOe in the temperature range of 300~2 K. Presented in Fig. 2 is the temperature dependence of the complex magnetic susceptibility in the form ofXmTversus T. At 300 K, theXmTvalue is 1.53599 cm3?K?mol-1.When the temperature decreases, theXmTfalls significantly.At 2 K, theXmTvalue is 0.02376 cm3?K?mol-1. Moreover, as seen in Fig. 2, in the temperature range of 300~150 K, the data of 1/Xm vs. Tare in linear relationship. The linear regression equation is 1/Xm= 0.292T+ 106.74, with the correlation coefficient being 0.9951. According to the Curie-Weiss law,Xm=C/(T-θ), the calculated Weiss constant (θ)is negative. These magnetic behaviors reflect that the title complex exhibits antiferromagnetism.
3. 3 Fluorescent and sensing properties
The solid-state fluorescent properties were measured at room temperature in the range of 220~750 nm. Both excitation and emission spectra are depicted in Fig. 3. As revealed in Fig. 3, the excitation peak is around 322 nm. When the excitation wavelength is 322 nm, the complex shows two sharp fluorescence emission bands at 595 and 618 nm,respectively. They are the characteristic emission bands of europium ions[25], corresponding to the transitions of Eu3+:5D0→7F1(595 nm) and5D0→7F2(618 nm), respectively.The ratio of the electric dipole transition peak at 618 nm to the magnetic dipole transition peak at 595 nm is 4.03. This large ratio justifies that the europium ion in the complex is not at the inversion center and that the monochromaticity of the complex is good[26]. Interestingly, no emission bands from the organic ligand were observed, so the effective luminescence sensitization of central Eu(III) ions comes from ligands[27].
In addition, we studied the effect of CrO42-on the fluorescence intensity of the title complex and found that CrO42-solution can quench the fluorescence. Under the same conditions, the same amount of the title complex was added to CrO42-solutions with different concentrations, and different fluorescence emission spectra were determined. As shown in Fig. 4, with the increase of CrO42-concentration, the fluorescence peak intensity at 595 and 618 nm decreases gradually.When studying the fluorescence peak intensity at 618 nm by changing the CrO42-solution concentration, we found that between the CrO42-concentration range of 0.00 and 1.50 mmol·L-1, the fluorescence intensity ratioI0/Iis linear with the concentration of CrO42-. In accordance with Stern-Volmer equationI0/I= 1 +KSV[CrO42-], whereI0is the fluorescence intensity in blank solution,Ithe fluorescence intensity in CrO42-solution and [CrO42-] the concentration of CrO42-, the linear equation isI0/I= 0.9945 + 300.52[CrO42-], the correlation coefficient is 0.9761 and the value ofKsvis about 300.52 mol-1·L.
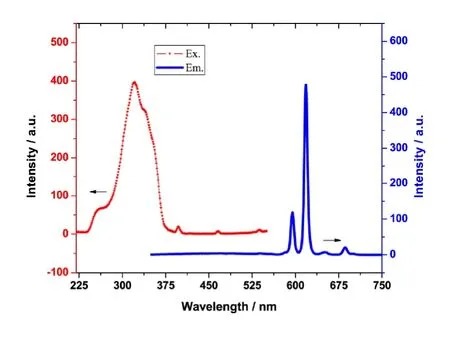
Fig. 3. Both excitation and emission spectra of the complex at room temperature
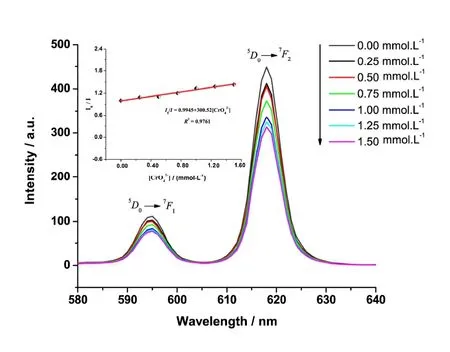
Fig. 4. Emission spectra of the complex in CrO42- solution with different concentrations (0.00~1.50 mmol·L-1)
- 結(jié)構(gòu)化學(xué)的其它文章
- Two Polynuclear Fe Complexes with Boat-like Core:Syntheses, Structures and Magnetic Properties①
- A Robust Heterometallic Cd(II)/Ba(II)-Organic Framework with Exposed Amino Group and Active Sites Exhibiting Excellent CO2/CH4 and C2H2/CH4 Separation①
- Synthesis, Crystal Structure, Spectroscopy and Hirshfeld Analysis of 4,6-Diamino-2-cyclopropylaminopyrimidine-5-carbonitrile with Different Solvents: N,N-dimethylformamide, Methanol and Water①
- Syntheses, Crystal Structures and DNA-Binding Properties of Zn(II) and Mn(II) Complexes Based on Imidazole Derivatives and Carboxylic Acid
- CoMFA Study on Anti-proliferative Activity of Fluoroquinolone Amide Derivatives①
- Mechanism Study of Aliskiren and Its Analogues by Molecular Dynamic Simulation①

