Feasibility of therapeutic endoscopic ultrasound in the bridge-to-surgery scenario: The example of pancreatic adenocarcinoma
Giuseppe Vanella, Domenico Tamburrino, Gabriele Capurso,Michiel Bronswijk,Michele Reni,Dell'Anna, Stefano Crippa, Schalk Van der Merwe, Massimo Falconi, Paolo Giorgio Arcidiacono
Abstract Upfront resection is becoming a rarer indication for pancreatic ductal adenocarcinoma, as biologic behavior and natural history of the disease has boosted indications for neoadjuvant treatments. Jaundice, gastric outlet obstruction and acute cholecystitis can frequently complicate this window of opportunity,resulting in potentially deleterious chemotherapy discontinuation, whose resumption relies on effective, prompt and long-lasting management of these complications. Although therapeutic endoscopic ultrasound (t-EUS) can potentially offer some advantages over comparators, its use in potentially resectable patients is primal and has unfairly been restricted for fear of potential technical difficulties during subsequent surgery. This is a narrative review of available evidence regarding EUS-guided choledochoduodenostomy, gastrojejunostomy and gallbladder drainage in the bridge-to-surgery scenario. Proof-ofconcept evidence suggests no influence of t-EUS procedures on outcomes of eventual subsequent surgery. Moreover, the very high efficacy-invasiveness ratio over comparators in managing pancreatic cancer-related symptoms or complications can provide a powerful weapon against chemotherapy discontinuation, potentially resulting in higher subsequent resectability. Available evidence is discussed in this short paper, together with technical notes that might be useful for endoscopists and surgeons operating in this scenario. No published evidence supports restricting t-EUS in potential surgical candidates, especially in the setting of pancreatic cancer patients undergoing neoadjuvant chemotherapy. Bridge-to-surgery t-EUS deserves further prospective evaluation.
Key Words: Endosonography; Gastrojejunostomy; Choledochoduodenostomy; Gallbladder drainage;Pancreatic cancer; Pancreatic surgery
lNTRODUCTlON
Jaundice, gastric outlet obstruction and acute cholecystitis (AC) can frequently complicate the clinical course of patients with pancreatic ductal adenocarcinoma (PDAC)[1 ]. Rapid, effective and long-lasting management of these events remains crucial to allow chemotherapy initiation or continuation.Traditional endoscopic or percutaneous palliation of these events may carry some limitations:Endoscopic retrograde cholangiopancreatography (ERCP) fails in up to 5 %-10 % of cases. Duodenal stenting is burdened by frequent symptoms recurrence. Percutaneous cholecystostomy (PT-GBD) is prone to tube dislodgement and cholecystitis recurrence[2 -4 ]. Therapeutic EUS (t-EUS) is showing increasing potential in overcoming some of these limitations. However, EUS-guided procedures have been conventionally restricted to inoperable patients, for fear of interference with eventual surgery.
In an era in which upfront resection of PDAC is decreasing in favor of a more frequent use of neoadjuvant chemotherapy, this prudential limitation might result in a significant subset of patients being excluded from the potential advantages of t-EUS[5 ]. The aim of this narrative review was to discuss available evidence on outcomes of t-EUS in the bridge-to-surgery scenario.
METHODS
A literature search was performed regarding EUS-guided biliary drainage, EUS-guided gastrojejunostomy (EUS-GJ) and EUS-guided gallbladder drainage (EUS-GBD) in the bridge-to-surgery scenario up to July 2020 . Available evidence was discussed in this narrative review, together with technical considerations and suggestions useful for endoscopists and surgeons operating in this setting. No original data are presented in the manuscript, and therefore Institutional Review Board approval was not required.Anonymized pictures are included from patients who have signed a specific written informed consent.
CLlNlCAL SCENARlO
In the combined treatment plan of PDAC, surgery is theoretically the only curative option, but only approximate 20 % of patients are surgical candidates at diagnosis, the remaining being metastatic or locally unresectable[6 ].
Even in resectable disease, increasing knowledge of PDAC biology has expanded criteria for neoadjuvant chemotherapy to control potential micrometastases and select the best surgical candidates[5 ,7 ,8 ]. Therefore, half of PDAC patients, with resectable, borderline resectable or locally advanced tumor, will start a chemo (radio) therapeutic regimen, being eventually considered for subsequent surgery in case of stable disease or partial response. This “window of opportunity” where cancer behavior is ascertained can last 6 mo or more. During this time symptoms need to remain palliated through minimally invasive procedures, providing long-term efficacy with low risk of dysfunction/recurrence, to optimize tolerance of oncological treatments.
POTENTlAL ADVANTAGES OF THERAPEUTlC EUS PROCEDURES OVER ALTERNATlVES
EUS is gaining ground in palliation of cancer symptoms, where the least possible invasiveness allows non-delayed oncological treatments, potentially leading to increased survival and prolonged time to quality of life deterioration. Biliary drainage might be required in more than half of patients with PDAC[9 ]. The gold standard approach (ERCP) might fail in up to 5 %-10 % of cases, as the papilla might be unreachable or cannulation may fail[2 ].
EUS-guided biliary drainage has an established role when ERCP fails, avoiding morbidity of percutaneous drainage[10 ]. Electrocautery-enhanced lumen apposing metal stents have improved simplicity and safety of EUS-guided choledochoduodenostomy (EUS-CD; Figure 1 ) to such an extent that this procedure is being proposed as an upfront alternative to ERCP in distal malignant obstruction,with the potential to reduce the rate of post-ERCP acute pancreatitis[11 ,12 ].
Although surgical gastrojejunostomy has shown better long-term results for gastric outlet obstruction, endoscopic placement of duodenal self-expandable metal stents is conventionally used as a first-line treatment[3 ]. This minimally invasive technique harbors some limitations, as it may provide suboptimal relief with high risk of gastric outlet obstruction recurrence[6 ].
EUS-GJ is currently investigated as an alternative to both enteral stenting and surgical bypass.Placement of an electrocautery-enhanced lumen apposing metal stent between the stomach and proximal jejunum results in a large (2 cm) surgical-like anastomosis at a significant distance from the tumor. This minimally invasive procedure (Figure 2 ) may avoid both adverse events of surgery and the risk of primary failure, recurrence and re-interventions following enteral stenting[13 -15 ].
Even if rarer, AC can also complicate the clinical history of PDAC patients, due to a neoplastic infiltration of the cystic duct, eventually worsened by other palliative maneuvers, such as the placement of a self-expandable biliary metal stent[16 -18 ]. When surgical cholecystectomy is undesirable or unfeasible,EUS-GBD (Figure 3 ) has demonstrated its advantages over PT-GBD, resulting in equal technical success,paired with reduced 30 -d and 1 -year risk of adverse events, reintervention rates and AC recurrence[4 ].Moreover, the large-diameter fistula between the gallbladder and the gastrointestinal tract allows for subsequent endoscopic clearance of gallstones, potentially offering a definitive solution for AC[19 ]. All these advantages might be even more valuable in the setting of patients undergoing neoadjuvant chemotherapy since a long-lasting, effective, minimally invasive palliation of cancer symptoms might result in reduced chemotherapy discontinuation and potentially to more frequent resectability.
EXlSTlNG EVlDENCE ON SURGERY AFTER THERAPEUTlC EUS
To date there is limited experience on surgery following t-EUS, which is confined to cholecystectomy after EUS-GBD and a small series of pancreaticoduodenectomy after EUS-CD. As for cholecystectomy after EUS-GBD, a retrospective multicentric case-control study analyzed outcomes of subsequent cholecystectomy in 34 patients previously undergoing EUS-GBD with LAMS vs PT-GBD[20 ]. The study showed no influence of previous drainage modality in technical success, rate of conversion to open laparotomy or post-surgical adverse events, further showing a reduced operative time in the EUS-GBD cohort and a significantly higher number of interval procedures between PT-GBD and cholecystectomy due to plastic catheter maintenance or dislodgement.
Fabbriet al[21 ] described 5 patients undergoing pylorus-preserving pancreaticoduodenectomy after EUS-CD with 100 % technical success and no biliary/duodenal fistula. In a recent study, Gaujouxet al[22 ] described 21 patients undergoing pancreaticoduodenectomy after EUS-CD with electrocauteryenhanced lumen apposing metal stents: all surgeries were successful, without any postoperative biliary fistula or stricture or tumor recurrence at the hepaticojejunostomy site.
We recently published the first pancreaticoduodenectomy following EUS-guided double bypass.Surgical identification and disconnection of EUS-GJ was easy and fast and subsequent hepaticojejunostomy and gastrojejunostomy uncomplicated[23 ].
ENDOSCOPlC TRlCKS FOR EUS-GUlDED PROCEDURES lN POTENTlALLY RESECTABLE PATlENTS
Endoscopists involved in t-EUS may wonder whether the technical approach to EUS-CD should change when treating potentially resectable patients. In our opinion, two principles must be kept in mind in this specific setting: EUS-CD should be performed: (1 ) Through the bulb; and (2 ) As far away from the hilum as possible. Although no evidence exists on the impact of these technical variables, a “distal” EUS-CD will allow more space for bile duct transection and hepaticojejunostomy (Figure 1 A). Regarding EUSCD location, a transbulbar (instead of transgastric) window nullifies the theoretical risk of tumor seeding since the bulb falls within surgical resection margins.
As for EUS-GJ, theoretical constraints are broader than the millimetric evaluation during EUS-CD.The EUS-GJ site must be kept as close as possible to the ligament of Treitz, in the first jejunal loop, to sacrifice a limited amount of jejunum during reconstruction. In our experience, this is obtained by careful evaluation of fluoroscopy during nasojejunal tube insertion. As previously described, we believe that puncture of the colon or too distant a loop can be avoided relying on EUS-guided transgastric visualization of the nasojejunal tube and fluid flow inside the jejunum[24 ]. For all these reasons, we never place the tip of the nasojejunal tube too far from the ligament of Treitz. Finally, in case both procedures are required, we have no concern in performing them during the same sedation, but we believe that sequence matters[24 ]. EUS-GJ must be performed first, as an effective gastroenteric conduit is fundamental for successful transluminal biliary drainage. Conversely, if EUS-GJ proves technically unfeasible, a correctly placed EUS-CD may be rendered dysfunctional, resulting in subsequent cholangitis[25 ].
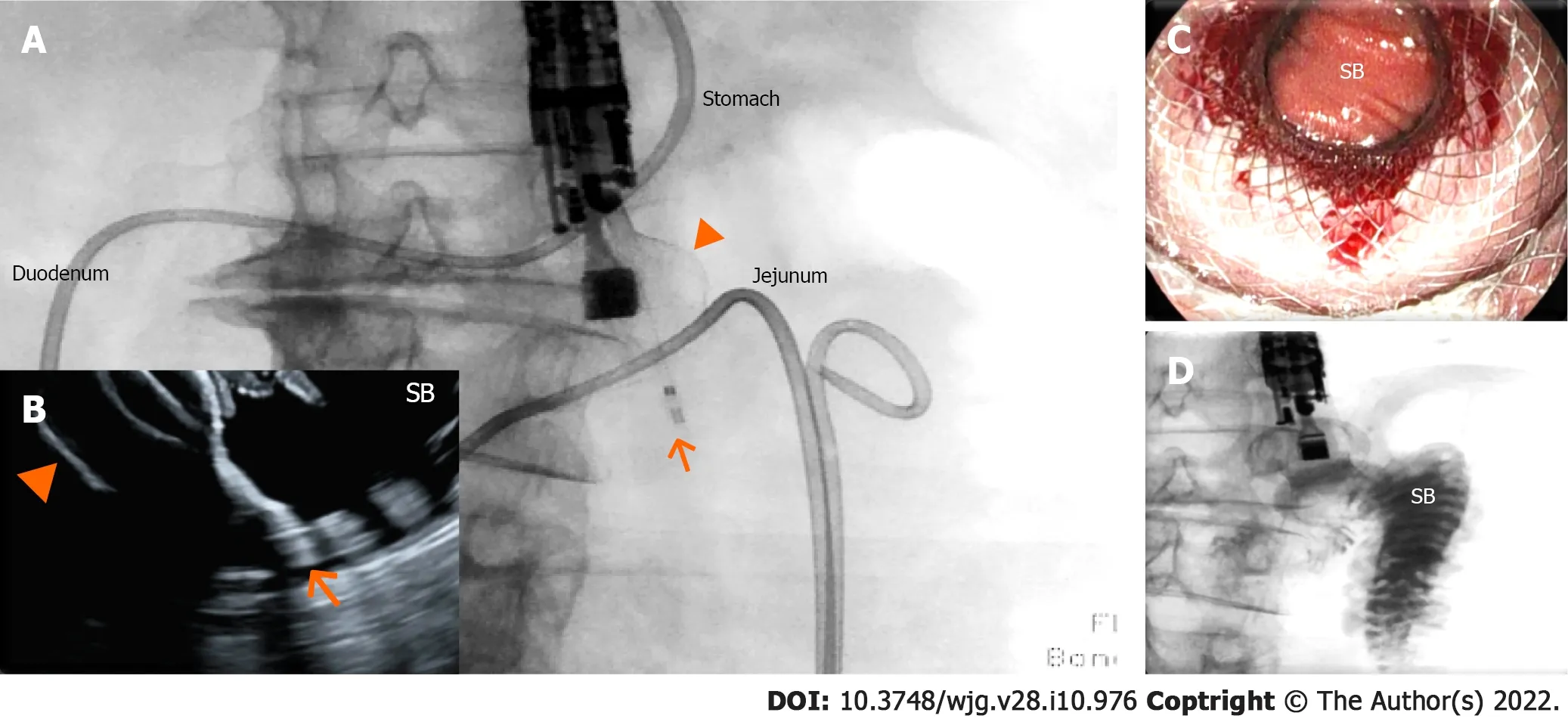
Figure 2 Endoscopic ultrasound-guided gastrojejunostomy. A and B: The small bowel has been distended with saline infusion through a nasojejunal tube. The dilated jejunal loop has been identified through the gastric wall by endosonography and has been accessed through a 20 mm enhanced lumen apposing metal stent (arrow: tip of the catheter; arrowhead: distal flange); under fluoroscopic (A) and endosonographic (B) guidance, demonstrating the opening of the distal flange (arrowhead) inside the small bowel (SB); C: The proximal flange of the electrocautery-enhanced lumen apposing metal stent has been released and dilated,and the SB can be visualized through the lumen apposing metal stent; D: Contrast injected through the nasojejunal tube can be aspirated through the lumen apposing metal stent inside the stomach.
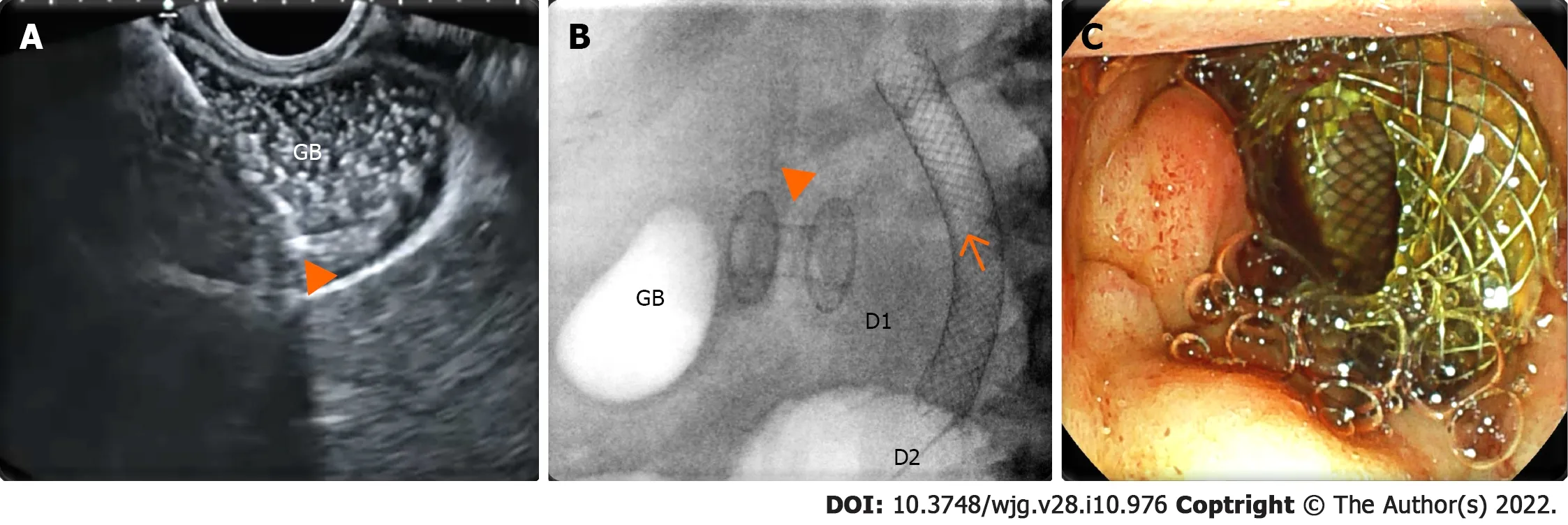
Figure 3 Endoscopic ultrasound-guided gallbladder drainage. A: Endosonographic view of the delivery system (arrowhead) of the enhanced lumen apposing metal stent inside a distended gallbladder (GB) full of sludge; B: Radioscopic view of the lumen apposing metal stent (arrowhead) released between the gallbladder and duodenal bulb [D1 ; biliary stent (arrow) released in the second duodenal portion (D2 )]; C: Endoscopic view of the proximal flange of the lumen apposing metal stent inside the duodenal bulb.
As for EUS-GBD with LAMS, one important consideration might regard the site for drainage. If EUSGBD is performed in a patient who is potentially a candidate to a pancreaticoduodenectomy, a transduodenal route for drainage makes the fistula lie within the resection margins of subsequent surgery. In all other kinds of surgeries (including simple cholecystectomy), a surgical revision of a cholecystogastrostomy is technically simpler than a cholecystoduodenostomy, and therefore the endoscopist should predilect a transgastric route when feasible. Moreover, all the attempts to reduce LAMS-related trauma must be pursued to avoid additional inflammatory reactions that might complicate surgery. We suggest the systematic placement of a short coaxial double-pigtail plastic stent inside the LAMS to avoid food impaction and to protect the contralateral wall from mechanical trauma,a lesson learnt from the management of peripancreatic fluid collections[26 ]. Furthermore, as described also in the setting of peripancreatic fluid collections, a longer stent indwelling might predispose to a higher risk of adverse events, and therefore we propose a systematic removal of the LAMS within 4 wk in case surgery must be postponed beyond this interval[27 ].
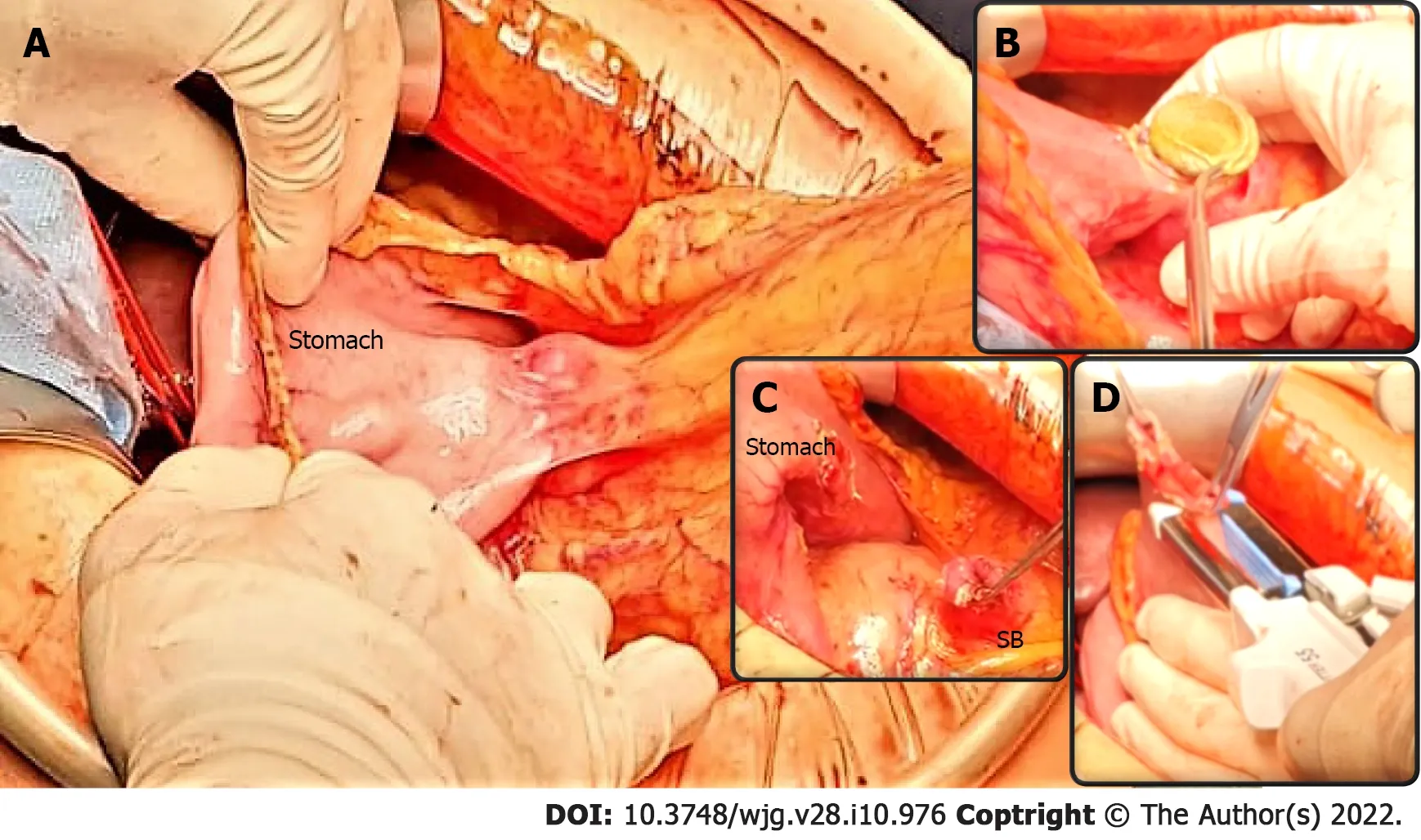
Figure 4 Pancreaticoduodenectomy following endoscopic ultrasound-guided double bypass. A: The endoscopic ultrasound-guided gastrojejunostomy site is easily identified and a stable gastrojejunal anastomosis is visible (underlined by blue curves) between the stomach and the small bowel(SB); B-C: The endoscopic ultrasound-guided gastrojejunostomy site is opened with diathermic coagulation, the lumen apposing metal stent is removed (B), and the anastomosis cut (C); D: The SB is prepared for gastroenteric anastomosis, while the gastric defect will be closed using staplers. A classic pylorus-preserving pancreaticoduodenectomy is achievable.
SURGlCAL TRlCKS FOR PANCREATlCODUODENECTOMY FOLLOWlNG EUS-GUlDED DOUBLE BYPASS
T-EUS is not a contraindication for PD. Usually the GJ anastomosis is performed between the posterior gastric wall and the fourth duodenal portion/first jejunal loop. As the first part of the resection we suggest identifying the EUS-GJ anastomosis (Figure 4 ), to open it with diathermic coagulation and to remove the LAMS. At this stage, the gastric wall defect can be closed using staplers, whereas small bowel will be resected as part of the pancreaticoduodenectomy. With this technique, a classic pyloruspreserving pancreaticoduodenectomy is achievable. As a second stage, we suggest performing a retrograde cholecystectomy in order to reach the common bile duct at the liver hilum. At this point, a proper lymphadenectomy of the liver hilum is performed, and the hepatic artery, portal vein and common bile duct are isolated and encircled with vessel loops. The common bile duct should be cut above the cystic duct’s confluence, and EUS-CD will therefore remain within resection margins and removed simultaneously.
In the case of former EUS-GBD, the approach will depend on the site of drainage and the type of surgery. If the patient is a candidate to a pancreaticoduodenectomy and the gallbladder was drained through the duodenum, the fistula remains within resection margins. In the case that the gallbladder was drained transgastric, the fistula can be transected as described for EUS-GJ. The gastric wall defect can be closed either using staplers or using suture with eventual omental protection.
CONCLUSlON
Upfront resection is becoming a rarer indication in PDAC patients, as biologic behavior and natural history of the disease has boosted indications for neoadjuvant treatment. An effective chemotherapy,capable of carrying the patient to eventual surgery, greatly depends on an effective and long-lasting palliation of disease-related gastrointestinal symptoms and complications. While conventional endoscopic/percutaneous palliation carries intrinsic disadvantages, t-EUS may overcome these limitations, without apparently increasing technical difficulty or complicating subsequent surgery. For all these reasons we believe that t-EUS should not be restricted in the bridge-to-surgery scenario while deserving further prospective evaluation in this setting (Figure 5 ).
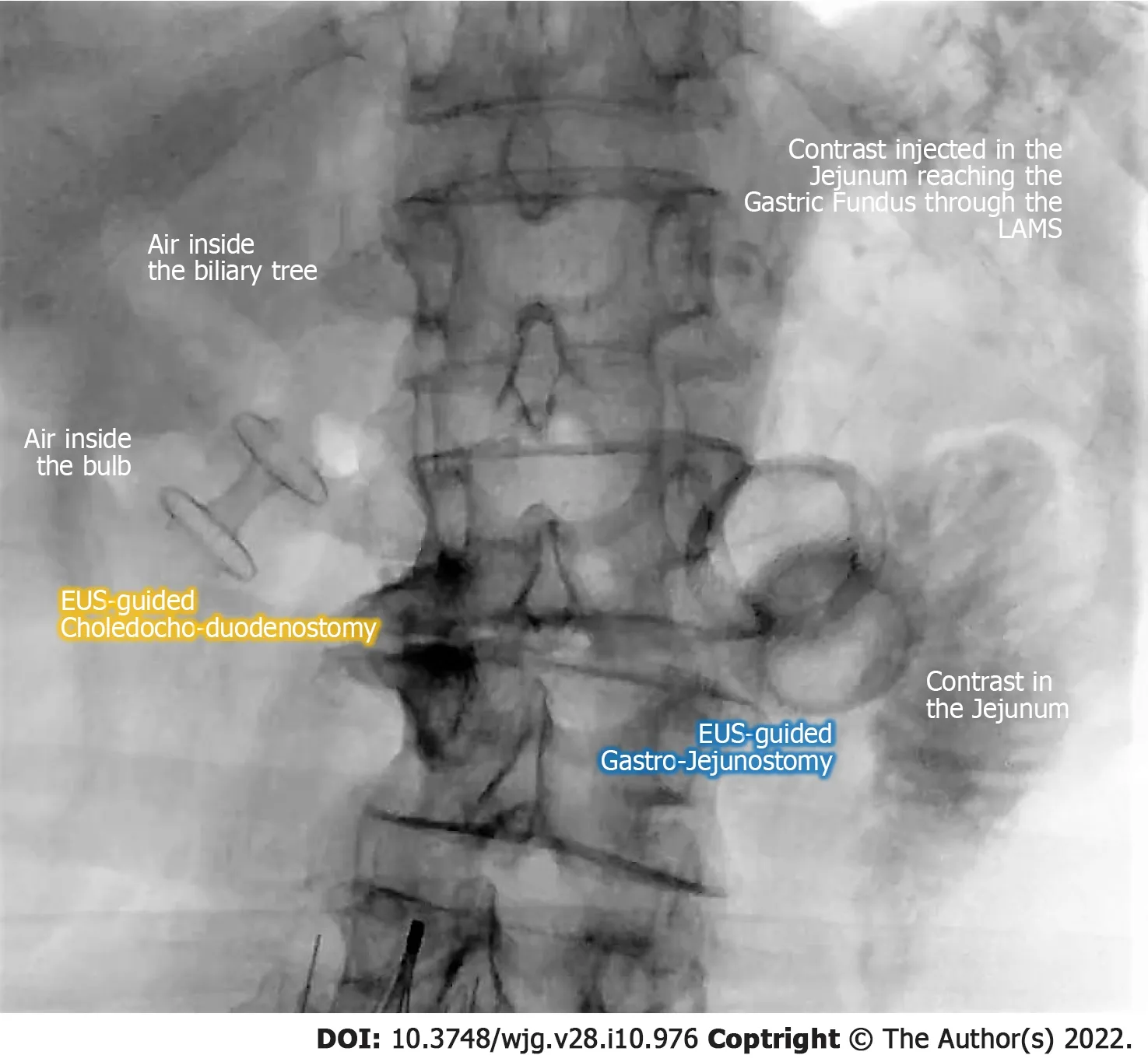
Figure 5 Fluoroscopic final image of an endoscopic ultrasound-guided double bypass with choledochobulbostomy and gastrojejunostomy. EUS: Endoscopic ultrasound; LAMS: Lumen apposing metal stent.
FOOTNOTES
Author contributions:Vanella G, Capurso G and Arcidiacono PG conceived the manuscript; Vanella G, Bronswijk M and Tamburrino D performed the literature search; Vanella G, Bronswijk M, Tamburrino D, Dell’Anna G and Capurso G drafted the paper; Arcidiacono PG, Reni M, Crippa S, Van der Merwe S and Falconi M critically revised the manuscript for important intellectual content; Vanella G created the figures; all authors agreed on the final submitted version, accepting to be accountable for accuracy or integrity of any part of the work.
Conflict-of-interest statement:Michiel Bronswijk has consultancy agreements with Prion Medical and Taewoong.Schalk van der Merwe holds the Cook Medical and Boston Scientific chair in Interventional Endoscopy and holds consultancy agreements with Cook Medical, Pentax and Olympus. The remaining authors declare no conflict of interest relevant for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: http://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:Italy
ORClD number:Giuseppe Vanella 0000 -0001 -7280 -1761 ; Domenico Tamburrino 0000 -0003 -1435 -3651 ; Gabriele Capurso 0000 -0002 -0019 -8753 ; Michiel Bronswijk 0000 -0003 -2039 -5022 ; Michele Reni 0000 -0002 -6463 -0293 ; Giuseppe Dell'Anna 0000 -0002 -9395 -5468 ; Stefano Crippa 0000 -0002 -7370 -1508 ; Schalk Van der Merwe 0000 -0002 -9891 -2686 ; Massimo Falconi 0000 -0001 -9654 -7243 ; Paolo Giorgio Arcidiacono 0000 -0001 -6692 -7720 .
S-Editor:Wu YXJ
L-Editor:Filipodia
P-Editor:Wu YXJ
 World Journal of Gastroenterology2022年10期
World Journal of Gastroenterology2022年10期
- World Journal of Gastroenterology的其它文章
- Comment “Asymptomatic small intestinal ulcerative lesions: Obesity and Helicobacter pylori are likely to be risk factors”
- Gut dysbiosis and small intestinal bacterial overgrowth as independent forms of gut microbiota disorders in cirrhosis
- Diagnostic performance of endoscopic classifications for neoplastic lesions in patients with ulcerative colitis: A retrospective case-control study
- Loss of LAT1 sex-dependently delays recovery after caerulein-induced acute pancreatitis
- Malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer
- An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms
