A Method for Determination of Critical Micellar Concentration of Surfactant
Zhang Nana
Suzhou Hybiome Biomedical Engineering Co.,Ltd,Jiangsu,China
Yang Jingbang
Suzhou New District Water Purification Co.,Ltd.Jiangsu,China
Zhang Guoquan
Suzhou Hybiome Biomedical Engineering Co.,Ltd.,China
Abstract Measure the chemiluminescence value of acridine compound as luminescent agent in the surfactant solution with a series of concentrations,according to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain several fitting lines,and the minimum value of the surfactant concentration corresponding to the intersection of the two adjacent fitting lines is the critical micelle concentration of the surfactant.According to the results,the critical micelle concentration of Dodecyl trimethyl ammonium chloride is 11.5 mmol/L,Hexadecyl trimethyl ammonium Bromide is 0.357 mmol/L,Sodium dodecyl sulfate is 7.64 mmol/L,sodium N-lauroylsarcosinate is 0.890 mmol/L,Triton X-100 is 0.309 mmol/L,3-((3-Cholamidopropyl)dimethylammonium)-1-propanesulfonate is 9.53 mmol/L,3-(N,N-dimethyldodecylammonio)propanesulfonate is 1.73 mmol/L.The results were similar to those obtained by traditional methods,the method can be used in the study of critical micelle concentration of surfactants.
Key words surfactant;critical micelle concentration;chemiLuminescence;acridine ester
There are many methods to determine the critical micellar concentration of surfactants,and the commonly used methods include surface tension method,conductivity method,dye method,turbidity method,fluorescence probe method,light scattering method[1].However,all these methods have their limitations.The conductivity method is only suitable for measuring the cmc of ionic surfactants.The dye method requires simple equipment to measure cmc,but the accuracy of cmc measurement is affected because the color change is not obvious enough.Sometimes,the addition of dye may affect the critical micelle concentration of the system,which may lead to wrong measurement results.Turbidity method also affected the accuracy of cmc measurement because of the influence of hydrocarbon solubles on the critical micelle concentration of surfactant.The fluorescence probe method is simple to operate and has no special requirements for the system.The amount of probe is small and the interference to the system is small.However,the benzene solution of pyrene probe needs to be prepared,which is toxic and the operation process is complicated.Light scattering method requires the solution to be very clean and has many limiting factors.A new method for determining the cmc of surfactants has been developed and the cmc of different types of surfactants has been tested.
1 Experiment
1.1 Equipment and materials
Nitric acid,30% hydrogen peroxide(AR),sodium hydroxide(AR),potassium hydroxide(AR),Dodecyl trimethyl ammonium chloride(DTAC)(98%),cetyl trimethyl ammonium bromide(CTAB)(98%),sodium dodecyl sulfate(SDS)(CP),sodium N-lauroyl sarcosine(98%),Sinopharm Chemical Reagent Co.,LTD.Triton X-100(AR),3-[3-(cholamide-propyl)dimethylamino]propanesulfonate(CHAPS),Lauryl dimethyl sulfobetaine(SB3-12),Sigma;Acridine ester marker for carcinoembryonic antigen,provided by Suzhou Hybiome Biomedical Engineering CO.,LTD.
Automatic chemiluminescence determination instrument(AE-180,A1800),Suzhou Hybiome Biomedical Engineering CO.,LTD.
1.2 Method
1.2.1 Preparation for buffer A
Measure the volume of 300 mL purified water,2.39 mL HNO3and 10.84 mL 30% H2O2.Add them into a wide-mouth glass container that is protected from light in order and add purified water to 500 mL.After stirring and mixing,the Buffer A was filtered and stored at room temperature.
1.2.2 Preparation for buffer B
Prepared a certain volume and concentration NaOH(KOH)solution;After dissolving and mixing a certain amount of surfactant with the above prepared solution,a original liquid is obtained;Dilute the original liquid to a series of concentration sample solutions with prepared NaOH(KOH)solution,which are stored at room temperature.
A series of DTAC conc.Buffer B:Dissolve the NaOH in water to prepare the 200ml 0.1 mol/L NaOH solution.Dissolve 1.0 g of DTAC with 100 mL NaOH solution and mix to obtain DTAC original liquid.Dilute the DTAC original liquid with the remaining NaOH solution to prepare a series of concentration sample solutions with a DTAC concentration of 0.1~ 50 mg/mL.
A series of CTAB conc.Buffer B:Dissolve the NaOH in water to prepare the 200 mL 0.2 mol/L NaOH solution.Dissolve 1.0g of CTAB with 100 mL NaOH solution and mix to obtain CTAB original liquid.Dilute the CTAB original liquid with the remaining NaOH solution to prepare a series of concentration sample solutions with a CTAB concentration of 0.0325~16.67 mg/mL.
A series of SDS conc.Buffer B:Dissolve the NaOH in water to prepare the 200 mL 0.5 mol/L NaOH solution.Dissolve 1.0 g of SDS with 100 mL NaOH solution and mix to obtain SDS original liquid.Dilute the SDS original liquid with the remaining NaOH solution to prepare a series of concentration sample solutions with a SDS concentration of 0.08~40 mg/mL.
A series of sodium N-lauroyl sarcosine conc.Buffer B:Dissolve the KOH in water to prepare the 200 mL 0.65 mol/L KOH solution.Dissolve 1.0 g of sodium N-lauroyl sarcosine with 100 mL KOH solution and mix to obtain sodium N-lauroyl sarcosine original liquid.Dilute the sodium N-lauroyl sarcosine original liquid with the remaining KOH solution to prepare a series of concentration sample solutions with a sodium N-lauroyl sarcosine concentration of 0.04~20 mg/mL.
A series of TX-100 conc.Buffer B:Dissolve the KOH in water to prepare the 200 mL 0.3 mol/L KOH solution.Dissolve 1.0g of TX-100with 100 mL KOH solution and mix to obtain TX-100 original liquid.Dilute the TX-100 original liquid with the remaining KOH solution to prepare a series of concentration sample solutions with a TX-100 concentration of 0.024 4~12.5 mg/mL.
A series of CHAPS conc.Buffer B:Dissolve the KOH in water to prepare the 200 mL 0.5 mol/L KOH solution.Dissolve 1.0 g of CHAPS with 100mL KOH solution and mix to obtain CHAPS original liquid.Dilute the CHAPS original liquid with the remaining KOH solution to prepare a series of concentration sample solutions with a CHAPS concentration of 0.1~25 mg/mL.
A series of SB3-12 conc.Buffer B:Dissolve the KOH in water to prepare the 200 mL 0.5 mol/L KOH solution.Dissolve 1.0 g of SB3-12 with 100 mL KOH solution and mix to obtain SB3-12 original liquid.Dilute the SB3-12 original liquid with the remaining KOH solution to prepare a series of concentration sample solutions with a SB3-12 concentration of 0.097 7~25 mg/mL.
1.2.3 Preparation for photondetection solution
Add carcinoembryonic antigen acridine ester marker to MES-BSA buffer to obtain acridine ester solution with a concentration of 2.4 mmol/mL.
1.2.4 Operation steps
At 20 ℃± 5 ℃,place the photondetection solution,buffer A and buffer B at the fixed position of the automatic chemiluminescence tester respectively.Turn on the photondetection mode,add 10 μL,2.4 mmol/mL acridine ester solution,150 μL buffer A and 150 μL buffer B is used to obtain the mixed solution,in which the pH of the mixed solution containing DTAC is 10.90,the pH of the mixed solution containing CTAB is 11.20,the pH of the mixed solution containing SDS is 11.60,the pH of the mixed solution containing sodium N-lauroyl sarcosine is 11.71,the pH of the mixed solution containing TX-100 is 11.38,the pH of the mixed solution containing CHAPS is 11.60,and the pH of the mixed solution containing SB3-12 is 11.60.Detect the luminescence signal value of the mixed solution for 5S respectively.A series of data are obtained according to the logarithm of the obtained signal value and the buffer B with different concentrations of each type of surfactant.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain several fitting lines,and the minimum value of the surfactant concentration corresponding to the intersection of the two adjacent fitting lines is the critical micelle concentration of the surfactant.
2 Results and discussion
2.1 CMC determination of different surfactants
2.1.1 Concentration and luminescence value of DTAC
DTAC solutions for 2.5,1.25,0.63,0.31,0.16,0.08,0.04,0.02,0.01,0.005 concentrations(g/mL)were prepared respectively.Turn on the photondetection mode,the luminescence values of the mixed solution for 5 s were detected in the optical test mode.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain fitting two lines.The DTAC concentration corresponding to the intersection of the two fitting lines is its cmc.According to Figure 1,the critical micelle concentration of DTAC was 0.304 g/mL,i.e.11.5 mmol/L[2,3].
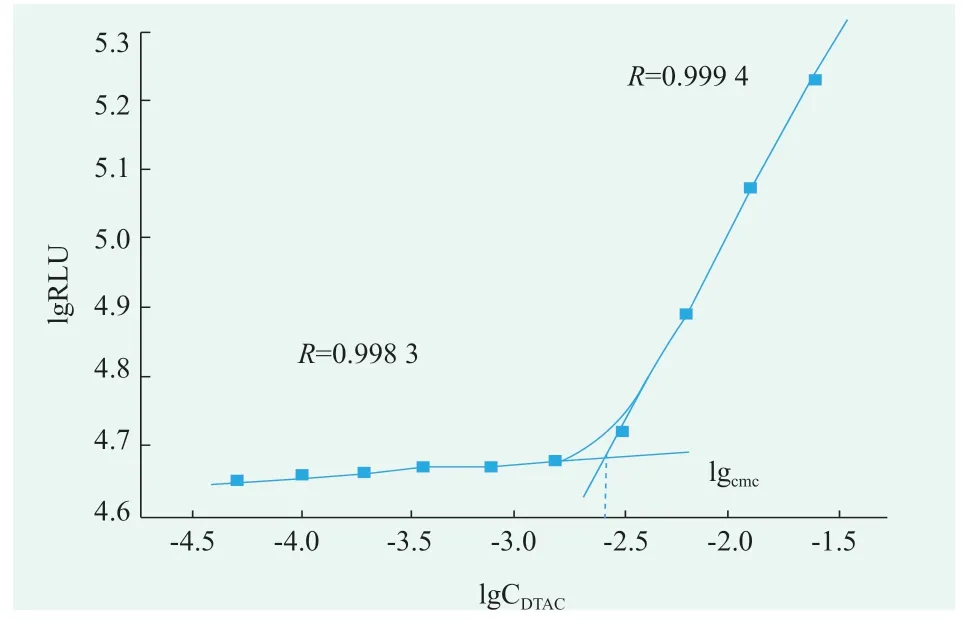
Figure 1.The critical micelle concentration of DTAC
2.1.2 Concentration and luminescence value of CTAB
CTAB solutions for 1.0,0.5,0.25,0.125,0.062 5,0.031 3,0.015 6,0.007 8,0.003 9,0.002 0,0.001 0,0.000 5 concentrations(g/mL)were prepared respectively.Turn on the photondetection mode,the luminescence values of the mixed solution for 5 s were detected in the optical test mode.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain fitting two lines.The DTAC concentration corresponding to the intersection of the two fitting lines is its cmc.According to Figure 2,the critical micelle concentration of CTAB was 0.013 g/mL,i.e.0.357 mmol/L[4-6].
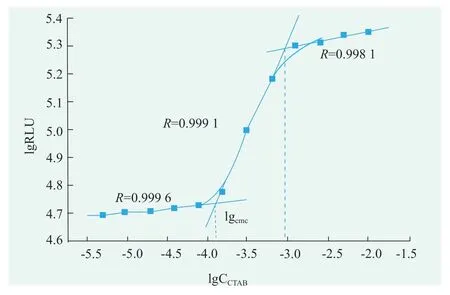
Figure 2.The critical micelle concentration of CTAB
2.1.3 Concentration and luminescence value of SDS
SDS solutions for 2.0,1.0,0.5,0.25,0.125,0.063,0.031,0.016,0.008,0.004 concentrations(g/mL)were prepared respectively.Turn on the photondetection mode,the luminescence values of the mixed solution for 5 s were detected in the optical test mode.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain fitting two lines.The SDS concentration corresponding to the intersection of the two fitting lines is its cmc.According to Figure 3,the critical micelle concentration of SDS was 0.22 g/mL,i.e.7.64 mmol/L[7-9].
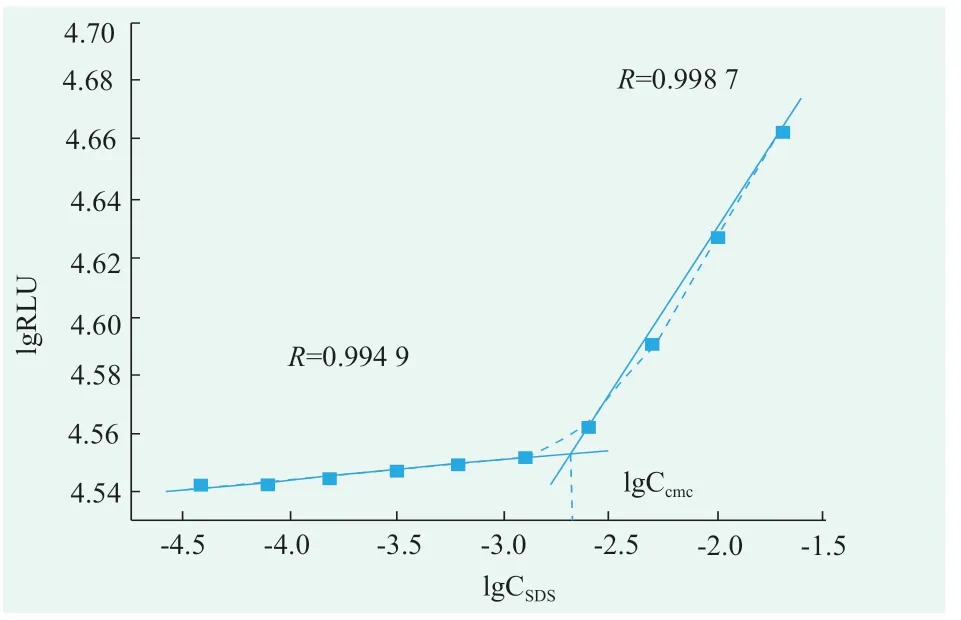
Figure 3.The critical micelle concentration of SDS
2.1.4 Concentration and luminescence value ofSodium N-lauroyl sarcosine
Sodium N-lauroyl sarcosine solutions for 0.5,0.25,0.125,0.063,0.031,0.016,0.008,0.004,0.002 0,0.001 0 concentrations(g/mL)were prepared respectively.Turn on the photondetection mode,the luminescence values of the mixed solution for 5 s were detected in the optical test mode.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain fitting two lines.The Sodium N-lauroyl sarcosine concentration corresponding to the intersection of the two fitting lines is its cmc.According to Figure 4,the critical micelle concentration of Sodium N-lauroyl sarcosine was 0.0261 g/mL,i.e.0.890 mmol/L[10].
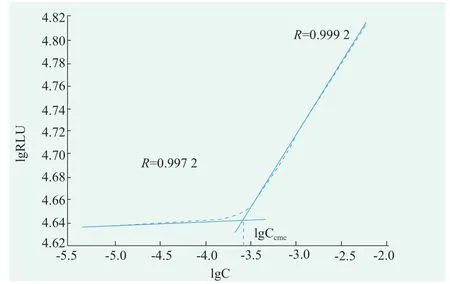
Figure 4.The critical micelle concentration of Sodium N-lauroyl sarcosine
2.1.5 Concentration and luminescence value of TX-100
TX-100 solutions for 0.625,0.313,0.156,0.078,0.039,0.020,0.01,0.005,0.002,0.001 concentrations(g/mL)were prepared respectively.Turn on the photondetection mode,the luminescence values of the mixed solution for 5 s were detected in the optical test mode.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain fitting two lines.The TX-100 concentration corresponding to the intersection of the two fitting lines is its cmc.According to Figure 5,the critical micelle concentration of TX-100 was 0.02 g/mL,i.e.0.309 mmol/L[11-14].

Figure 5.The critical micelle concentration of TX-100
2.1.6 Concentration and luminescence value of CHAPS
CHAPS solutions for 3.0,1.5,0.75,0.38,0.19,0.09,0.05,0.02,0.01,0.005 concentrations(g/mL)were prepared respectively.Turn on the photondetection mode,the luminescence values of the mixed solution for 5 s were detected in the optical test mode.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain fitting two lines.The CHAPS concentration corresponding to the intersection of the two fitting lines is its cmc.According to Figure 6,the critical micelle concentration of CHAPS was 0.586 g/mL,i.e.9.53 mmol/L[15,16].
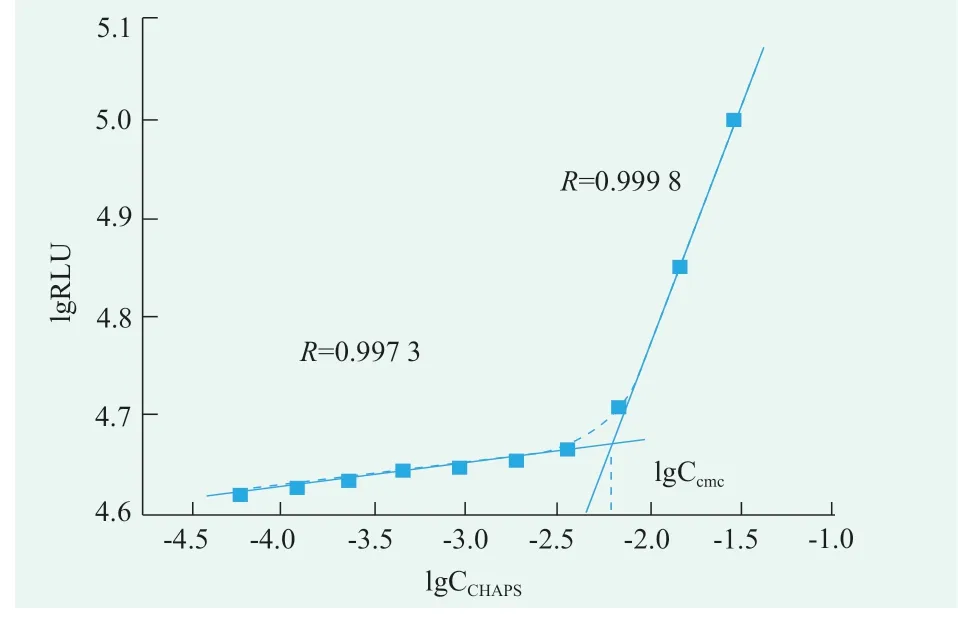
Figure 6.The critical micelle concentration of CHAPS
2.1.7 Concentration and luminescence value of SB3-12
SB3-12 solutions for 3.0,1.5,0.75,0.38,0.19,0.09,0.05,0.02,0.01,0.005 concentrations(g/mL)were prepared respectively.Turn on the photondetection mode,the luminescence values of the mixed solution for 5 s were detected in the optical test mode.According to the value of the obtained luminescent value and the value of the corresponding surfactant concentration in the surfactant solution,linear fitting is carried out to obtain fitting two lines.The SB3-12 concentration corresponding to the intersection of the two fitting lines is its cmc.According to Figure 7,the critical micelle concentration of SB3-12 was 0.057 9 g/mL,i.e.1.73 mmol/L[17].
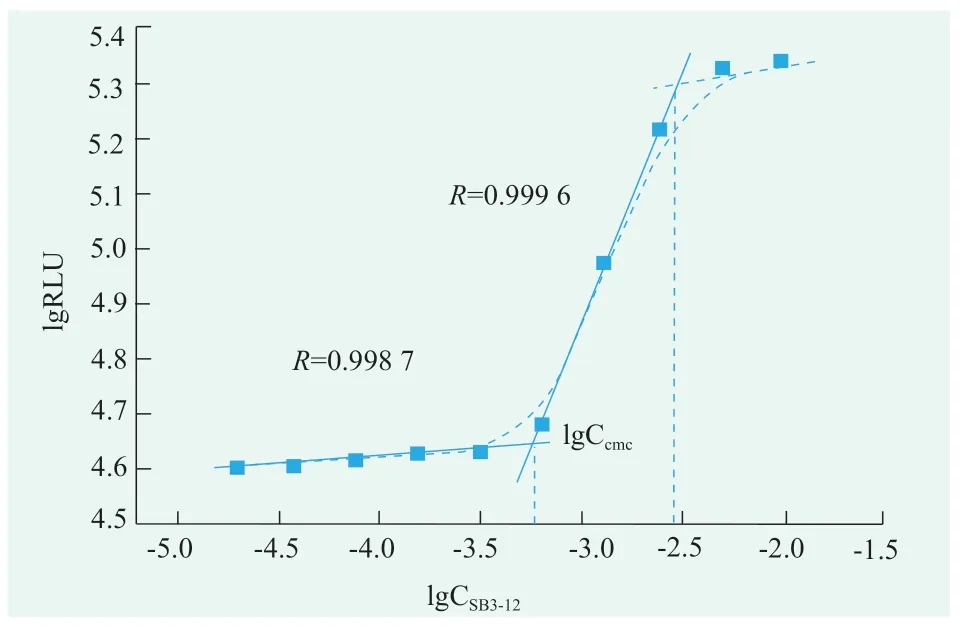
Figure 7.The critical micelle concentration of SB3-12
3 Methodological comparison
The cmc of the surfactants tested in this paper was compared with the data in the literature,and the results were analyzed in Table 1.
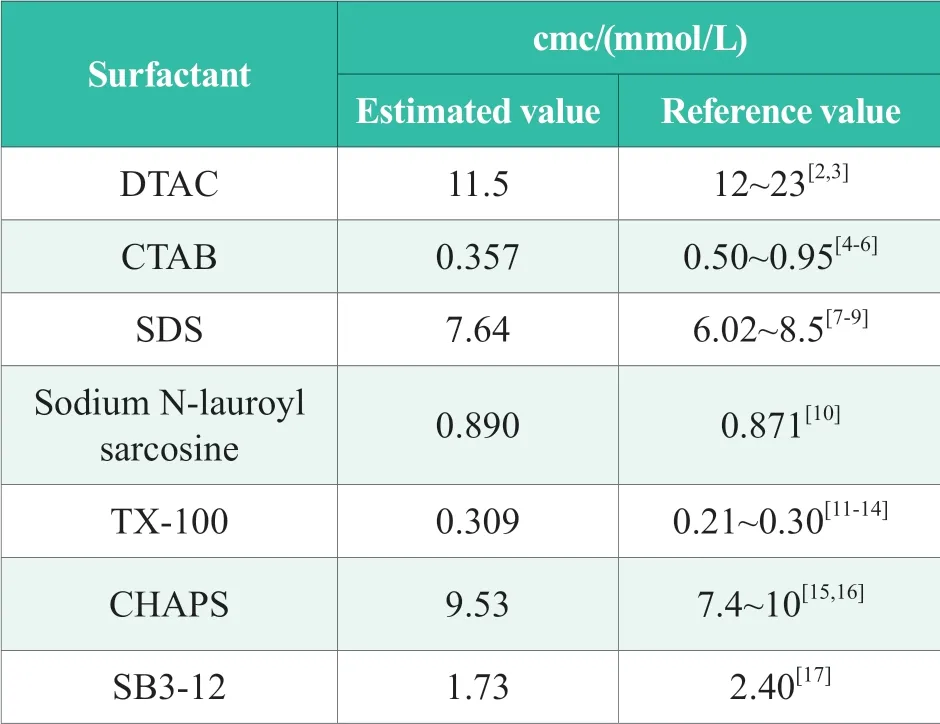
Table 1.Results of determination of critical micellar concentration of surfactant
In the Table 1,the results obtained by this method are very close to cmc data in current references,indicating that this chemiluminescence analysis method is a new method for determining the critical micelle concentration of surfactants.
3 Discussion
In this study,acridine esterification chemiluminescence system is characterized by high sensitivity and high accuracy.By its luminescence signal value detection,can quickly obtain the critical micelle concentration of surfactant,and compares with the traditional methods,the result shows that testing results of the critical micelle concentration data in this study method are equivalent to the testing data referenced from literatures,the results are relatively similar,indicating that the chemiluminescence analysis method is a kind of determination of surfactant and measuring method of the critical micelle concentration.
All indicators can meet clinical application requirements.This method can be widely used in the study of critical micellar concentration of surfactant.
 China Detergent & Cosmetics2022年1期
China Detergent & Cosmetics2022年1期
- China Detergent & Cosmetics的其它文章
- “R&D Competitions” of New Prominent Beauty Makeups
- Makeup Mirror Becoming the New Blue Ocean of Beauty Industry
- Application of Water-Based Polyurethane in Sunscreen Products
- Analysis and Comparison of Cleaning Power and Moisturizing Effect of Different Shower Gels
- Study on the Factors Affecting the Darkening of Liquid Foundations
- Experimental Method of Cosmetics Human Efficacy Evaluation Whitening
