MOLECULAR CHARACTERIZATION OF A SCLEROSTIN HOMOLOGUE IN BARBEL STEED (HEMIBARBUS LABEO) AND ITS RELATIONSHIP WITH INTERMUSCULAR BONE DEVELOPMENT
CHEN Jie, LIU Zi-Ming, JIANG Wei and Lü Yao-Ping
(College of Ecology, Lishui University, Lishui 323000, China)
Abstract: Previous studies have demonstrated that sclerostin (SOST) binds to and antagonizes the activity of bone morphogenetic proteins (BMPs). Because of the potential role of BMPs in intermuscular bone (IB) development, we explored the relationship between SOST and IB development. In this study, we identified a cDNA sequence encoding a SOST homologue in barbel steed (Hemibarbus labeo). Sequence analysis revealed that the SOST protein is composed of a signal peptide and a mature peptide which contained a C-terminal cystine knot-like domain. Phylogenetic tree analysis indicated that barbel steed SOST was closely related to goldfish (Carassius auratus) SOST. Expression of SOST transcripts were detected in all the tested tissues, with the highest transcript levels being found in the gill. in situ RNA hybridization results showed that SOST mRNA was obviously distributed in the myosepta. RT-qPCR showed that the transcript levels of the SOST gene significantly changed during the four developmental stages of IBs. This timing overlapped with IB formation, providing support for a role of SOST in IB development.
Key words: Barbel steed; SOST; Gene expression; Intermuscular bone; Ossification
It is well known that most freshwater fish in the world, especially Cyprinidae species, have a certain number of intermuscular bones (IBs), which are hardboned spicules located in the muscle tissue on both sides of the vertebra[1,2]. The development of IBs has been described as undergoing intramembranous ossification of connective tissue in the myosepta between neighboring myomeres[3,4]. This process is initiated by mesenchymal stem cells, an unspecialized cell population that can develop into osteoblasts[5]. The presence of IBs does substantially affect the meat quality and commercial values of many cyprinid fishes in aquaculture. In view of the potential value in eliminating IBs, researchers began to study IBs in fish as early as the 1960s[6]. But so far, few studies have been published on the molecular mechanism of IBs formation, so the understanding of the homology and origin of fish IBs is mainly based on morphological data[2,7].
Sclerostin (SOST) is a glycoprotein that plays an important role in bone formation and the regulation of osteoblastic activity[8]. In mammals, SOST is mainly expressed in mature osteocytes where SOST plays a role in bone regulation[9]. However, SOST mRNA has also been found in the heart, liver, and kidney although the function in these tissues is not known[9]. In addition, SOST has been proved to inhibit BMP-stimulated bone formation through antagonizes Wnt signaling in osteoblastic cells[10]. Analysis of SOST knockout mice revealed an inhibitory action of SOST on Wnt signaling in both osteoblasts and osteocytes[9].Most recently, BMP genes has been shown that play potential roles in IBs formation and maintenance in fish[11,12], suggesting that SOST gene may also be in-volved in IBs development. However, until now,mammalian homologs ofSOSTgene have been identified; the studies of theSOSTgene in lower vertebrates are rare[13—15].
Barbel steed (Hemibarbus labeo) is a freshwater bottom dwellers cyprinid living in streams and feeding on aquatic insects. It is distributed in major drainage of East Mainland China, Japan, and Korea and the species has been an important farmed fish in China. However, like other cyprinid fishes, barbel steed also has many IBs. The morphological characteristics, emergent periods and morphogenesis of IBs in barbel steed has been studied in our previous research[16—18]. In order to investigate molecular mechanism of IBs formation, we cloned theSOSTgene from barbel steed. We also determined the distribution of SOST and the association between its expression and IB development. Moreover, the four key stages related to IB development in barbel steed were determined using Alizarin red staining.
1 Materials and methods
1.1 Fish maintenance and sample collection
Barbel steed larvae were reared in our laboratory at Lishui University. Larvae were reared at 22—24℃ and fed with commercial feed. Larvae were collected from four key stages for IB development,which were identified by Wan,et al[19]. For bone staining andin situRNA hybridization, larvae were anesthetized with 0.1 μg/μL of tricaine methanesulfonate (MS-222), and were fixed in 4% paraformaldehyde (PFA). In order to analysis of temporal expression patterns ofSOSTgene during IBs development,carefully tore off the skin from tail and took the muscle (between the 35th to 40th myomere) under an anatomical lens. Then, larvae muscle tissue containing IBs in the tail were frozen in liquid nitrogen and stored at -80℃. In addition, adult fish tissues, including the liver, kidney, spleen, gill, heart, brain, muscle,skin, and intestine were also collected for analysis of the expression patterns in different tissues. Four samples from each tissue were collected, frozen in liquid nitrogen, and stored at -80℃ until being used.All experiments were performed in accordance with the Experimental Animal Management Law of China and were duly approved by the Animal Ethics Committee of Lishui University.
1.2 Sequencing analyses of SOST cDNA
The cDNA sequence of barbel steed SOST was obtained from the transcriptome data of barbel steed muscle. The authenticity of this cDNA was confirmed by specific amplification using reverse transcription polymerase chain reaction (RT-PCR) followed by cloning and sequencing. The similarity of the obtained sequence with other known sequences was assessed via BLAST searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple alignments were analysed using Clustal W (http://clustalw.ddbj.nig.ac.jp/).Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 6.
1.3 Alizarin red staining
To verify the four key stages related to IBs development in barbel steed, we performed Alizarin red staining according to a published protocol[16]. In brief,after being washed thrice with PBS to remove the 4%PFA, the samples were washed in TBST (50 mmol/L Tris pH 7.4, 150 mmol/L NaCl, 0.1% [v/v]Triton X-100) for 30min. Then placed in 1% trypsin (1∶250,BBI, Shanghai, China) for digestion and transferred to 2 mg/mL Alizarin red (Sangon, Shanghai, China) in 1% KOH for 1h followed by incubation in a series of glicerol-1% KOH bath (starting at 1∶3 ratio and increasing until 3∶1). Storage in absolute glycerol was only initiated when the specimens were completely clear. Ten microlitres of alkaline-equilibrated phenol were added to glycerol to prevent contamination. Specimens were observed and photographed using a conventional dissecting microscope SMZ1500 (Nikon,Tokyo, Japan), and taken photographs using NIS-Element D software (Nikon).
1.4 Gene expression analysis by RT-qPCR
Total RNAs were extracted from tissues using RNAiso reagent (TaKaRa, Dalian, China). After treatment with DNase I (TaKaRa), the first cDNA strand was synthesized using AMV reverse transcriptase(TaKaRa). Specific primer pair, SOST-t(+) (5′-AG GAGGTCATCAGCCAACTC-3′) and SOST-t(-) (5′-TGGCATGAGATGAGCTGGAA-3′), was used to amplify a 159 base pair (bp) fragment from SOST cDNA (MN306524). Primer pair, 18S rRNA-t(+) (5′-AGAAACGGCTACCACATCCA-3′) and 18S rRNA-t(-) (5′-CCGAGATCCAACTACGAGCT-3′), was used to amplify a 247 bp fragment from 18S rRNA(MH843153)[20]. The qPCR assay was performed with SYBR premix Ex Taq (Perfect Real Time; TaKaRa)using an ABI StepOne Real-Time PCR System (Applied Biosystems, Foster City, USA). The qPCR amplification conditions were as follows: 95℃ for 5min and 40 cycles at 95℃ for 30s, 60℃ for 30s, and 72℃for 30s, followed by melting curve analyses. The cycle threshold (Ct) values of SOST were normalized to those of 18S rRNA using the 2-ΔΔCtmethod[21].
1.5 Probe synthesis and in situ RNA hybridization
Specific primer pair, SOST-i(+) (5′-ACGAA TAAACCCGAGGAGCA-3′) and SOST-i(-) (5′-GGAAGGCATTGACCAGAACAC-3′), was used to amplify a 212 base pair (bp) fragment from SOST cDNA. Fragments were cloned into the pGEM-T vector (Promega, Madison, WI, USA) and used as the template to create a DIG-labeled riboprobe, using RNA polymerases and labeling mix (Roche, San Francisco, USA).
Forin situhybridization, tail (between the 35th to 40th myomere) of barbel steed were frozen on dry ice and sectioned at 14 μm. All sections were stored on polylysine coated slides at -80℃, before being oven-dried. Then, sections were subjected to proteinase K (10 μg/mL) treatment. Hybridisation was performed in humidified conditions for 16h at 58℃with DIG-labelled probe added (0.4 μg/mL). After hybridization, sections were incubated with an anti-DIG antibody (1∶5000), and stained with NBT/BCIP(Roche). Sections were observed and photographed using a dissecting microscope (SMZ1500, Nikon) and photographed using NIS-Element F 3.2 software(Nikon).
1.6 Statistical analysis
All data are presented as mean±SEM. Statistical analysis of the results was conducted using one-way ANOVA using SPSS version 13.0 (SPSS Inc, Chicago, USA). Statistical significance was considered atP<0.05.
2 Results
2.1 Molecular characterization of barbel steed SOST
The cDNA sequence of SOST has been submitted to the GenBank database under the accession number of MN306524. The cDNA contained an open reading frame (ORF) of 636 nt, which was predicted to encode a 211-amino acid (aa) polypeptide with a calculated molecular weight (MW) of 24.0 kD and isoelectric point (pI) of 10.08. The SOST protein is composed of a signal peptide and a mature peptide which contained a C-terminal cystine knot-like(CTCK) domain. The mature peptide of SOST has a putative MW of 20.9 kD and apI of 10.15. An alignment of barbel steed SOST with selected teleost SOST proteins showed that the CTCK domain is highly conserved in teleosts. In addition, SOST contains seven cysteine residues that are conserved in all the fish SOST homologues examined.
The phylogenetic tree analysis indicated that barbel steed SOST was first clustered with the goldfish SOST, and then these two fishes were clustered with zebrafish into a branch. At last, all fish SOSTs were clustered together and were distinct from mammalian group (Fig. 1).
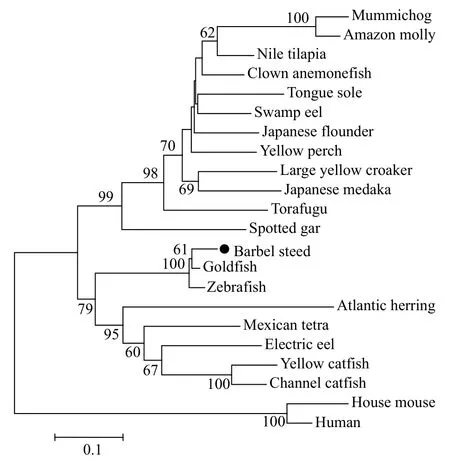
Fig. 1 Phylogenetic (neighbour-joining) analysis results of complete amino acid sequences of SOST using the MEGA6.0 program
The values at the forks indicate the percentage of trees in which this grouping occurred after bootstrapping (1000 replicates; shown only when>60%). The scale bar shows the number of substitutions per base.Accession numbers of the sequences used are as follows: electric eel (XM_027005976.1); torafugu(XM_003961038.3); yellow perch (XM_028566929);large yellow croaker (XM_010743662.3); yellow catfish (XM_027137499); Nile tilapia (XM_003448667);tongue sole (XM_008314410); zebrafish (XM_001340647); mummichog (XM_012879489); clown anemonefish (XM_023270202); Japanese flounder(XM_020109582); swamp eel (XM_020616499);spotted gar (XM_006638116); Atlantic herring (XM_012814585); Amazon molly (XM_016660803); Mexican tetra (XM_007259138); channel catfish(XM_017484385); goldfish (LC382461.1); Japanese medaka (XM_004080635); house mouse (NM_024449);human (NM_025237).
2.2 Constitutive expression of barbel steed SOST gene
The constitutive expression ofSOSTgene was assessed in different tissues of adult barbel steed using RT-qPCR. The expression of theSOSTgene was detected in all tested tissues. The expression level of theSOSTgene was the highest in the gill, followed by the skin, and relatively low levels ofSOSTgene expression were detected in other tissues. We found that the expression level of theSOSTgene in the gill was 74.65-fold higher than that in the muscle (Fig. 2).
2.3 Development characteristics of IBs in the four development stages of barbel steed
In order to verify the four key stages related to IBs development in barbel steed, we stained larvae with Alizarin red (Fig. 3). Four development stages of IBs can be observed in barbel steed. StageⅠ: at 29 day after hatching (DAH) (body length≈17 mm), all of the bones in barbel steed were ossified except for the IBs (Fig. 3A); Stage Ⅱ: at 35 DAH (body length≈21 mm), a few IBs of small length have emerged in the tail (Fig. 3B); Stage Ⅲ: 42 DAH (body length≈24 mm), more IBs of greater length gradually emerged in the tail (Fig. 3C); Stage Ⅳ: at 57 DAH(body length≈3.0 mm), all of the IBs in the tail have a mature morphology and length (Fig. 3D). At the same time, IBs have also emerged throughout the body of the fish from the tail to the head.
2.4 Spatial expression patterns of the SOST gene during IB development
To determine the spatial and temporal expression pattern ofSOSTgene during the four developmental stages of IBs, we performedin situRNA hybridization. It is found that the signal is obviously distributed in the myosepta of the fish at stage I. With the development of the fish, the signal gradually weakened. At stage Ⅳ, the signal in the myosepta disappeared (Fig. 4).

Fig. 2 RT-qPCR was performed to analyse the constitutive expression of SOST in different tissues (liver, kidney, spleen, gill,heart, brain, muscle, skin, and intestine) of barbel steed

Fig. 3 Development characteristics of IBs in the four key development stages of barbel steed
2.5 Temporal expression patterns of SOST gene during IBs development
The temporal expression profiles ofSOSTgene during four developmental stages of IBs were analyzed by using RT-qPCR. We found that theSOSTgene expression decreased during the four developmental stages of IBs, and theSOSTgene expression decreased significantly during the transition from stage Ⅰ to stage Ⅱ, when IBs began to ossify. The expression level of SOST is then maintained at a lower level from stage Ⅱ to stage Ⅳ, at which time the IB formation is of fast growth and the size of existing IBs are become bigger (Fig. 5).

Fig. 4 Distribution of SOST gene during IB development

Fig. 5 Temporal expression patterns of SOST gene during IBs development
3 Discussion
SOST is a highly conserved, secreted, cystineknot protein which regulates osteoblast function.Mammalian homologs ofSOSTgene have been identified whereas the studies of theSOSTgene in lower vertebrates are rare. In the present study, the cDNA sequence of a putativeSOSTgene was identified in barbel steed. Sequence analysis results showed that SOST protein is composed of a signal peptide and a mature peptide which contained a CTCK domain.SOST inhibits osteoblastic bone formation by antagonizing Wnt signaling[10]. Mutations in the CTCK domain of the SOST reduced its ability to bind to low-density lipoprotein receptor-related protein 5 (LRP5)and inhibit Wnt signaling[22], suggesting CTCK is an important functional domain in SOST.
Barbel steed SOST is constitutively expressed in all the tested healthy fish tissues, such as, gill, skin,brain, and spleen. This is similar to the results observed in mammals, in addition to expression in bone,it is also expressed in other tissues, although the function in these tissues is not known[9]. Recently, SOST was detected in the developing nervous system and in Kupffer’s vesicle of zebrafish embryo[23], suggesting that SOST may play a role in modulating signals important for formation of the nervous system.
Previous studies have demonstrated that SOST binds to, and antagonizes the activity of the bone morphogenetic proteins[24,25], and because of the potential role of BMPs in the development of IBs in blunt snout bream (Megalobrama amblycephala)[11,12],We tried to explore the relationship between SOST and IBs development. In our study,in situRNA hybridization results showed that mRNA ofSOSTgene is obviously distributed in the myosepta of the fish at stage I. With the development of the fish, the signal gradually weakened. Similar to the result ofin situRNA hybridization, the result of RT-qPCR showed that the highest expression level of SOST was observed at stage I of IBs development, and theSOSTgene expression decreased significantly during the transition from stage Ⅰ to stage Ⅱ, when IBs began to ossify. The expression level of SOST is inversely related to the growth rate of bone. The lower the expression levels of SOST, the faster the bone development rate. The expression level of SOST is maintained at a lower level from stage Ⅱ to stage Ⅳ, at which time the IB formation is of fast growth and the size of existing IBs are become bigger. However, the expression level of SOST is positively related to the growth of IBs in crucian carp (Carassius auratus)[13],which may be due to the difference in sampling location causing the opposite trend ofSOSTgene expression[14]. Anyway, this timing overlapped with IBs formation, providing further support for a role for SOST in IBs development.
In conclusion, we characterized a fishSOSTgene from barbel steed. The temporal and spatial expression of theSOSTgene significantly changed during the four developmental stages of IBs. The findings presented in this study provide a basis for further investigations of SOST and evaluation of the role of SOST in the regulation of IB development in Cyprinidae species.

