Heterometallic Ln(Na)-MOFs(Ln=Tb,Dy,Ho):Crystal Structures,Luminescent Sensing for Acetaldehyde,Fe3+,Cr2O72-,and Electrochemical Sensing for Catechol
CHAI Hong?MeiYAN Jia?LingREN Yi?Xia*,GAO Lou?Jun*, ZHANG Gang?QiangZHANG YanHAO Shu?Yuan
(1Shaanxi Key Laboratory of Chemical Reaction Engineering,College of Chemistry and Chemical Engineering,Yan'an University,Yan'an,Shaanxi 716000,China)
(2Xinjiang Xuanli Environmental Protection Energy Co.,Ltd.,Hami,Xinjiang 839000,China)
Abstract:Three new 3D microporous isostructural heterometallic metal organic frameworks Ln(Na)?MOFs:{[LnNa(BDT)(H2O)3]·2H2O}n(Ln=Tb(1),Dy(2),Ho(3),H4BDT=3,5?bis(3′,5′?dicarboxylphenyl)?1H?1,2,4?triazole),have been synthesized under solvothermal condition,and characterized by single crystal X?ray diffraction,elemental analysis,thermogravimetric analysis,and powder X?ray diffraction.Structural analysis demonstrates that they possess the same 3D frameworks based on similar heteronuclear bimetallic units.Fluorescence studies show that Tb(Na)?MOF(1)can detect Fe3+ion,Cr2O72-anion,and acetaldehyde molecules with high sensitivity and selectivity by luminescent sensing,and can also be used for electrochemical detection of catechol in water.CCDC:2077995,1;2083696,2;2083700,3.
Keywords:heterometallic metal organic frameworks;crystal structure;luminescent sensing;electrochemical sensing
0 Introduction
With the rapid development of industry,toxic and harmful substances(including heavy metal ions,toxic small molecules,etc.)are increasingly harmful to the environment and human health.Therefore,the detec?tion of toxic and harmful substances plays an indis?pensable role in life sciences,biomedical and environ?mental science.Until now,various analytical tech?niques,such as atomic absorption spectrometry(AAS),atomic fluorescence spectrometry(AFS),inductively coupled plasma mass spectrometry(ICPMS),X?ray fluorescence spectroscopy(XRF),have been used for determination of toxic and harmful substances[1?2].However,complicated sample preparation,expensive instruments and high cost make these analytical meth?ods inconvenient[3].Therefore,the detection of toxic and harmful substances in aqueous solutions by a sim?ple method is significant for human health and ecologi?cal environment.
Compared with transition metals,lanthanide met?als have unique properties of 4forbitals.The electrons in the 4forbital can undergof?forf?dtransitions,pre?senting extremely rich energy level transitions and absorbing or emitting light of a very wide range of wave?lengths from the ultraviolet to the infrared region[4].So,the luminescent lanthanide MOFs(Ln?MOFs)have especial advantages in the field of fluorescence probes due to their excellent optical properties such as high color purity,large Stokes shift,sharp line emission and relatively long fluorescence lifetime,which can provide very favorable conditions for the design of modern high efficiency luminescent materials[5?7]. Until now,Ln?MOFs have been widely employed for the detection of inorganic ions and anions,organic(or inorganic)small molecules,antibiotics,neurotransmitters,explo?sives and temperature sensing[8?14].
In this study,we used 3,5?bis(3′,5′?dicarboxylphe?nyl)?1H?1,2,4?triazole(H4BDT,Scheme 1)to design and construct three isostructural heterometallic MOFs(Ln(Na)?MOFs),namely{[LnNa(BDT)(H2O)3]·2H2O}n(Ln=Tb(1),Dy(2),Ho(3),by self?assembly under sol?vothermal conditions.Ln(Na)?MOFs were characterized by single crystal X?ray diffraction,elemental analysis,thermogravimetric analysis(TGA),IR and powder X?ray diffraction(PXRD).Because of the antenna effect of rare earth complexes and the synergetic effect of organic ligands and central metal ions,Tb(Na)?MOF(1)showed excellent luminescent properties,and can detect Fe3+,acetaldehyde molecules and Cr2O72-.In addition,1 doped carbon paste electrode(1/CPE)had excellent electrochemical activity for sensing and iden?tifying catechol.
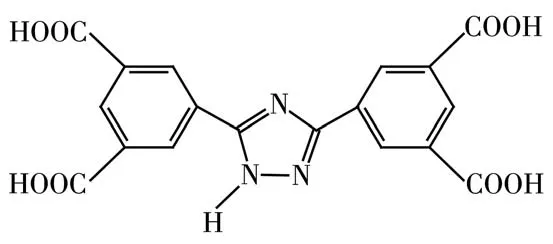
Scheme 1 Structure of H4BDT
1 Experimental
1.1 Materials and methods
Lanthanide metal salts(Tb(NO3)3·6H2O,DyCl3·6H2O,HoCl3·6H2O),H4BDT and otherchemical reagents were commercially purchased and didn′t require further purification.Elemental analyses of C,N,and H were performed on an Elementar VarioⅢtype element analyzer(Elementar Company in Germa?ny).IR(KBr pellet)spectra were recorded on a Shimad?zu IRAFFINITY?1S spectrometer in a range of 4 000?500 cm-1.Thermogravimetric measurements were per?formed on a ZPY?2P Thermal Analyzer(Shanghai Kaip?ing Instrument Factory)from 30 to 900 ℃ with a heat?ing rate of 10℃·min-1under N2atmosphere.PXRD patterns were collected on a PANalytical X′Pert PRO instrument with MoKαradiation(λ=0.154 06 nm,U=45 kV,I=40 mA,2θ=5°?30°).Fluorescent data were collected on a Hitachi F?7000 fluorescence spectrome?ter at room temperature.Cyclic voltammetry(CV)measurements were performed on a CHI660D Electro?chemical Workstation.
1.2 Synthesis of Ln(Na)-MOF
A mixture of lanthanide metal salts(0.05 mmol),H4BDT(0.05 mmol),4,4′?bipyridine(0.05 mmol),(CH3)2CHOH(1 mL)and HAc?NaAc buffer solution(5 mL,pH=6)were sealed in a 25 mL Teflon?lined stainless?steel autoclave.Then it was heated in an oven at 160℃for 3 d,and was cooled to room temperature at a descent rate of 4℃·h-1.Colourless crystals of Tb(Na)?MOF(1)were obtained and washed with water and ethyl alcohol,then dried in air(63%yield based on Tb).Anal.Calcd.for C18H17O13N3NaTb(%):C,32.48;H,2.56;N,6.32.Found(%):C,32.88;H,2.80;N,6.30.FT?IR(KBr pellet,cm-1):3 400(m),1 629(m),1 560(s),1 413(s),1 362(s),1 120(w),1 025(w),929(w),875(w),667(w),600(w).The IR spectrum is shown in Fig.S1(Supporting information).
Primrose yellow crystals of Dy(Na)?MOF(2)were obtained and washed with water and ethyl alcohol,then dried in air(78%yield based on Dy).Anal.Calcd.for C18H17O13N3NaDy(%):C,32.31;H,2.54;N,6.28.Found(%):C,32.82;H,2.45;N,6.33.FT?IR(KBr pellet,cm-1,Fig.S1):3 391(m),1 631(m),1 560(s),1 418(s),1 364(s),1 110(w),1 024(w),928(w),877(w),670(w),602(w).
Light pink crystals of Ho(Na)?MOF (3)were obtained and washed with water and ethyl alcohol,then dried in air(86%yield based on Ho).Anal.Calcd.for C18H17O13N3NaHo(%):C,32.19;H,2.53;N,6.26.Found(%):C,32.52;H,2.40;N,6.32.FT?IR(KBr pellet,cm-1,Fig.S1):3 384(m),1 627(m),1 559(s),1 419(s),1 363(s),1 109(w),1 022(w),927(w),878(w),670(w),599(w).
1.3 X-ray crystallography
Single crystal with regular morphology and high crystallinity were selected by optical microscope.The single crystal diffraction data were collected by Bruker D8 Venture system with MoKαradiation(λ=0.071 073 nm)at 296 K.The collected diffraction data were absorption?corrected usingSADABS program.The structure were solved by dual methods using SHELXT and refined by full?matrix least?squares methods againstF2.Crystallographic data and structure refine?ment for 1?3 are displayed in Table 1.The selected bond lengths and bond angles for Ln(Na)?MOF are sum?marized in Table S1.
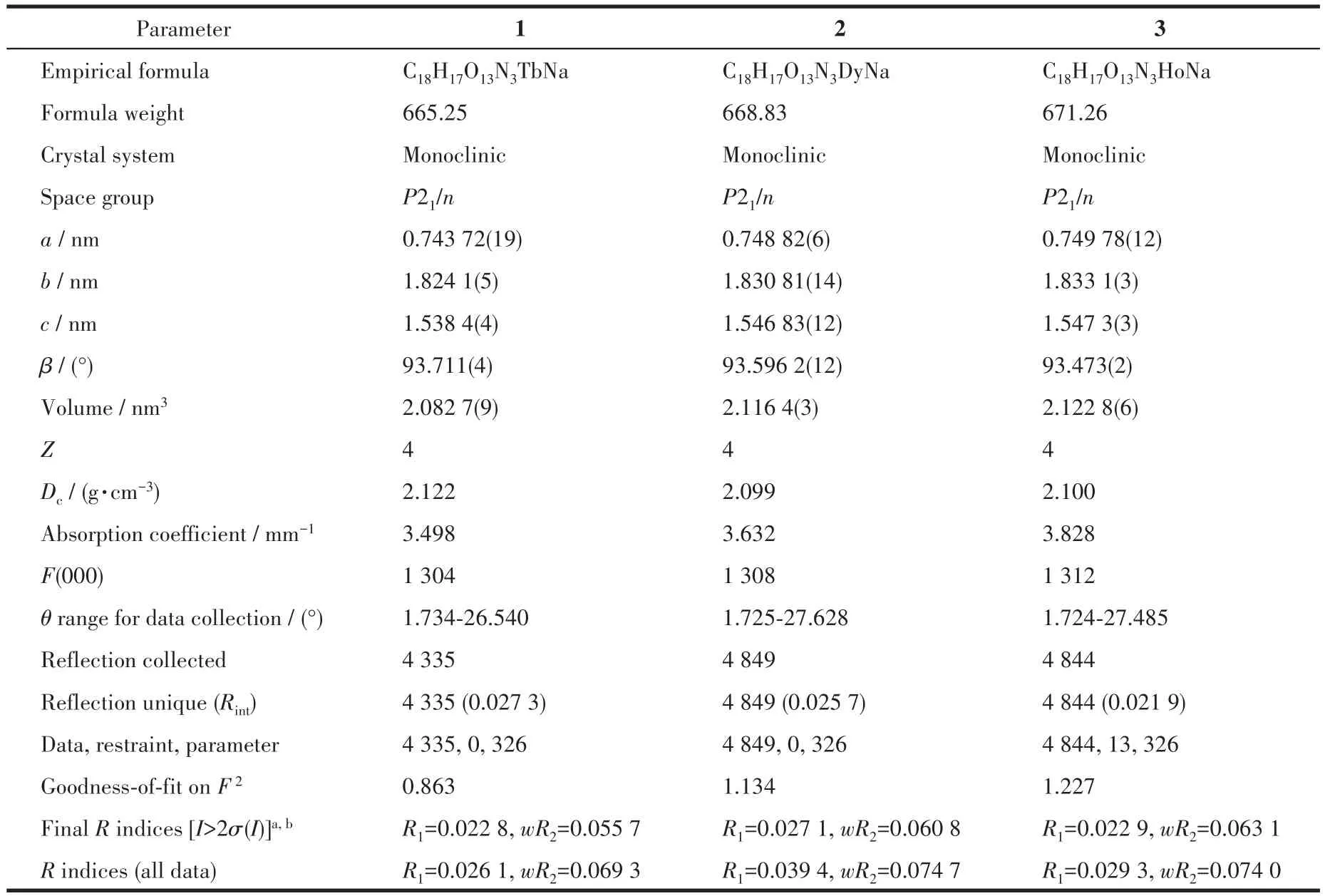
Table 1 Crystallographic data for 1-3
CCDC:2077995,1;2083696,2;2083700,3.
1.4 Fluorescence sensing experiment
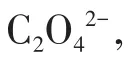
1.5 Electrochemical sensing experiment
MOF 1 was ground into fine powder using an agate mortar.Accurately obtained 10 mg powder of 1,3 g graphite powder,0.5 g liquid paraffin were thor?oughly mixed.Then,the 1/CPE electrode was prepared by filling the polyvinyl chloride(PVC)pipe with an inner diameter of 3 mm with compacting and inserting copper wire as the conductor.It was left at room tem?perature for a week and the CPE electrode was pol?ished to a mirror surface on the weighing paper before it was used.In phosphate buffered saline(pH=7),the electrochemical activity of 1/CPE was investigated by CV(sweep rate:0.02 mV·s-1)to detect theo?dihydroxy?benzene(catechol)in water with different concentra?tions(c=0.02?0.085 mmol·L-1).
2 Results and discussion
2.1 Crystal structure descriptions of 1-3
Crystallographic analyses show that complexes 1?3 crystallize in the monoclinic system with the space group ofP21/n(Table 1).Because the complexes exhib?it isomorphic 3D coordination frameworks with hetero?nuclear bimetallic units,so only the structure of 1 is described herein as a representative.The asymmetric unit of 1 contains one Tb3+ion,one Na+ion,one BDT4-ligand,three coordinated water molecules,and two lattice water molecules with the formula of[TbNa(BDT)(H2O)3]·2H2O.One Tb3+ion in the complex coordinates with seven oxygen atoms(O1,O3c,O4c,O5a,O6a,O7b,O8b)of four BDT4-ligands and two oxygen atoms(O9 and O10)from two water molecules to form a nine?coordinated configuration(Fig.1a).Among them,O4c,O5a and O8b are located at the caps of the three flanks of the distorted triangular prism,respectively,O1,O7b and O9 are the three vertices of the upper surface,and O3c,O6a and O10 are the three vertices of the lower surface of the triangular prism.Six carboxylic oxygens(O3c and O4c,O5a and O6a,O7b and O8b)are the chelating coordination,respectively,while an oxygen atom(O1)is the bridging coordination.At the same time,O5a and O7b also coordinate with Na+ion as bridging oxygen,connectting Tb3+and Na+into a het?eronuclear bimetallic unit(the distance of Tb…Na ions is 0.383 77 nm).Na+ion forms a five?coordinated con?figuration with an oxygen(O5a)in a water molecule and four atoms in BDT4-ligands,among them,the car?boxyl oxygen O2d,O7b and O11 of three BDT4-ligands and the N atom of one BDT4-are all bridging coordina?tion.And O1 on the carboxyl group acts as a bridging carboxyl group to connect the heteronuclear bimetallic units into a 1D chain(Fig.1b).The 1D chain is connect?ed by the flexible BDT4-to form a 2D two?layer plane structure(Fig.1c),then it expands into a 3D micropo?rous structure(Fig.1d).The bond lengths and bond angles of 1 are shown in Table S1.The Tb—O and Na—O bond lengths are in the ranges of 0.232 5(4)?0.252 1(3)nm and 0.223 4(4)?0.247 3(6)nm,respec?tively.The Na—N bond length is 0.251 6(5)nm,and the O—Tb—O and O—Na—N bond angles are in the ranges of 76.11(13)°?162.57(19)°and 89.41(19)°?108.98(16)°,respectively.These bond lengths conform to the reported ranges of Tb—O bond length and O—Tb—O bond angle[15].In 1?3,with the increase of atom?ic number from Tb to Ho,the average Ln—O bond lengths decreased from 0.243 7 to 0.242 9 nm gradual?ly due to the lanthanide contraction effect[16].So the average Na—O and Na—N bond lengths increased gradually from 0.234 9 to 0.236 9 nm and from 0.251 6 to 0.252 4 nm,respectively(Fig.1d?1f).
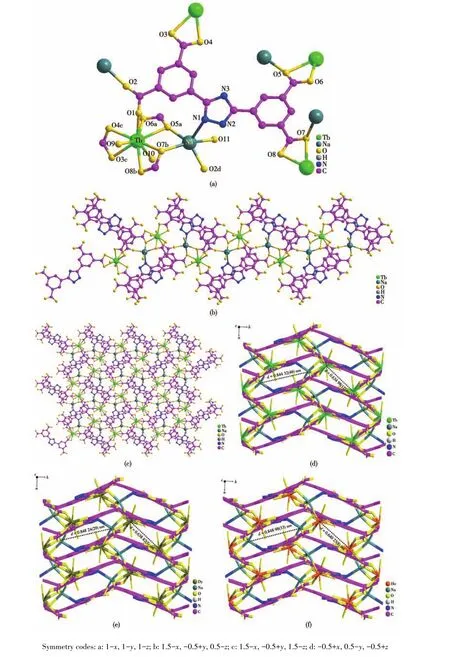
Fig.1 (a)Coordination environment of 1;(b)1D chains of heteronuclear bimetallic 1;(c)2D plane of 1;(d?f)3D structures of 1?3,respectively
2.2 Thermal stability and PXRD analysis
The TGA curves of 1?3 have been recorded(Fig.S2).The first weight loss of the MOFs ranging from 30 to 200℃were 13.0%for 1(Calcd.13.5%),13.46%for 2(Calcd.13.46%),13.46%for 3(Calcd.13.4%),which should be attributed to the weight loss for all the coordination and lattice water molecules.The second weight loss of MOFs 1?3 correspond to the slow decom?position of the framework over 470℃.PXRD patterns of 1?3 are shown in Fig.S3.Most peak positions in sim?ulated and experimental patterns were consistent with each other,indicating that 1?3 have good phase purity.
2.3 Luminescence characteristic in solid state
The solid state fluorescence spectra of H4BDT and 1?3 has been measured at room temperature under the excitation of 325 nm(slits:5 nm,PMT voltage:350 V)(Fig.2).Only MOF 1 showed four well resolved charac?teristic emission peaks at 490.6,544.6,584.8,and 621.6 nm,corresponding to typicalf?ftransitions of Tb3+ions,namely5D4→7F6,5D4→7F5,5D4→7F4,and5D4→7F3.Under the same condition,compared with 1,the fluorescence intensity of H4BDT,2,and 3 were negligi?ble.Therefore,the intensity change of the strongest emission peak(544.6 nm)was used as the detection index for analysis.
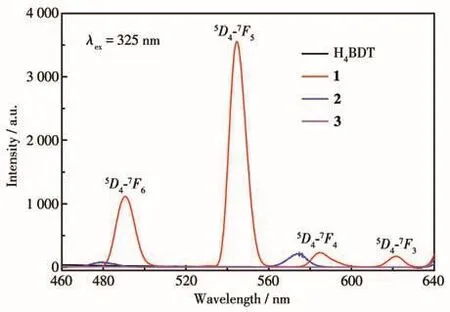
Fig.2 Solid?state fluorescence spectra of H4BDT,1,2,and 3 at room temperature
2.4 Fluorescent sensing of Tb(Na)-MOF
2.4.1 Sensing experiments for small organic molecules
The fluorescence spectra of 1 in different solvents showed that the luminescence intensity of 1 was differ?ent(Fig.S4),and the fluorescence quenching phenome?non took place in AH(Fig.3).The UV?Vis absorption of 1 in different solvents was recorded(Fig.S5).Com?pared to other solvents,the UV?Vis absorption band of AH had an obvious peak at 278 nm.Therefore,the fluo?rescence quenching of 1 by AH may be caused by com?petitive absorption.
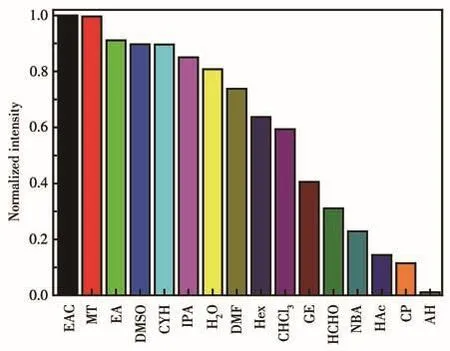
Fig.3 Relative fluorescence intensity of 1 in different solvents
To better understand the luminescence responses of AH to 1,the relationships between the concentra?tions of AH and the luminescence intensities were stud?ied as shown in Fig.4.The luminescence intensity of 1 gradually decreased with the increase of AH concentra?tion,presenting a first order exponential relationship:y=3 966.35exp(-x/83.837)+327.699(R2=0.993 94).When the concentration of AH was in a range of 5?100 μmol·L-1,there was a linear relationship(Fig.4 inset)between the concentration of AH and the luminescence intensity of 1:y=3 970.5-26.57x(R2=0.998 16);at the same time the detection limit(LOD)calculated was 0.125 μmol·L-1calculated by formula 3σ/k.Compared with reported documents for detection AH by lumines?cent MOFs(LOD:0.000 58%,about 102.7 μmol·L-1)[17],MOF 1 showed more excellent characteristics in terms of sensitivity.
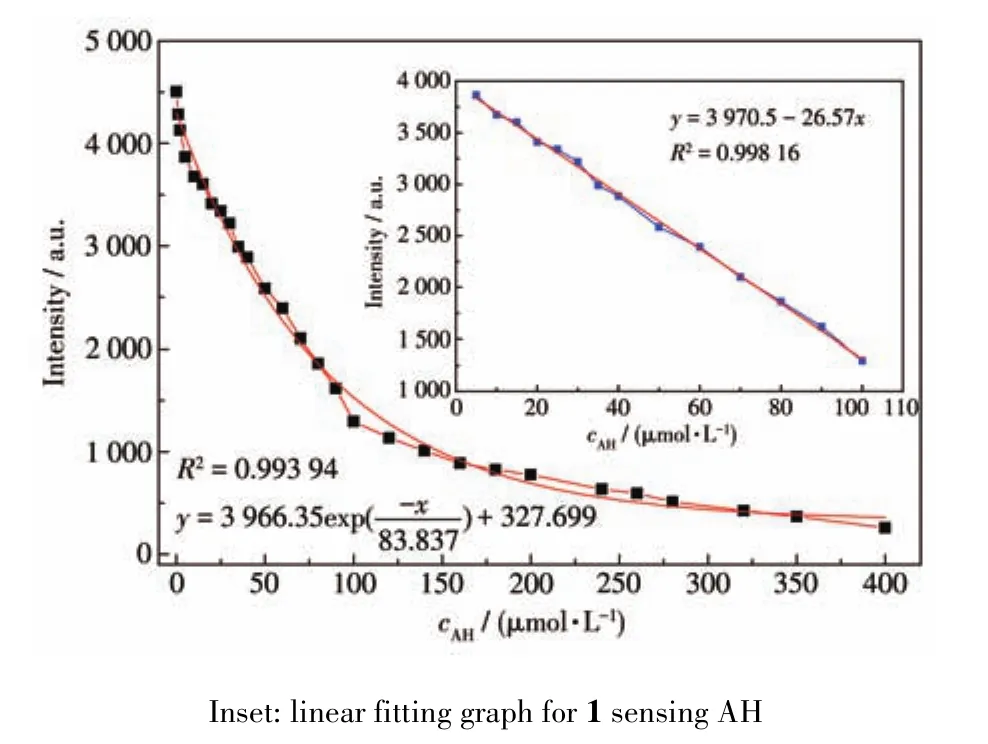
Fig.4 Nonlinear fitting graph for 1 sensing AH
2.4.2 Sensing experiments for metal ions
The fluorescence spectra of 1 in the presence of different metal ions are shown in Fig.S6.It is found that different metal ions had different effects on the flu?orescence intensity of 1.Especially in the solution of Fe3+,the phenomenon of fluorescence quenching was most obvious.Therefore,MOF 1 may be a good sensing material for detecting Fe3+ion(Fig.5).To better know the fluorescence quenching mechanism,the UV?Vis absorption spectra of different metal ions was recorded(Fig.S7).Compared to other metal ions,the UV?Vis absorption band of Fe3+had an obvious peak at 325 nm.Therefore,the fluorescence quenching of 1 by Fe3+may be caused by competitive absorption[18].
The luminescence intensity of 1 decreased gradu?ally when the concentration of Fe3+was changed from 10-11to 10-2mol·L-1(Fig.5,inset),and there was a good nonlinear relationship between the luminescence intensity of1 and the concentration ofFe3+,y=2 897.2exp[-x/(3.84×10-6)]+1 555.7exp[-x/(1.35×10-4)]+209.97(R2=0.994).
2.4.3 Sensing experiments for anions
The fluorescence spectra of 1 in different anion aqueous solutions was recorded(Fig.S8).The most interesting feature was that the fluorescence intensity of the inorganic anions incorporated 1 suspension was heavily dependent on the species of inorganic anions.Especially,Cr2O72-anion showed a drastic quenching effect on the luminescence of 1.While other inorganic anions have no significant quenching effect,indicative of the fact that 1 can be considered as a promising luminescent probe for Cr2O72-anion(Fig.6).The UV?Vis spectra of different inorganic anions was recorded(Fig.S9).Compared to other inorganic anions,the UV?Vis absorption band of Cr2O72-had an obvious peak between 310 and 400 nm.Therefore,this unique quenching effect for 1 possibly results from competitive absorption[19].

Fig.6 Relative fluorescence intensity of 1 in the presence of different anions
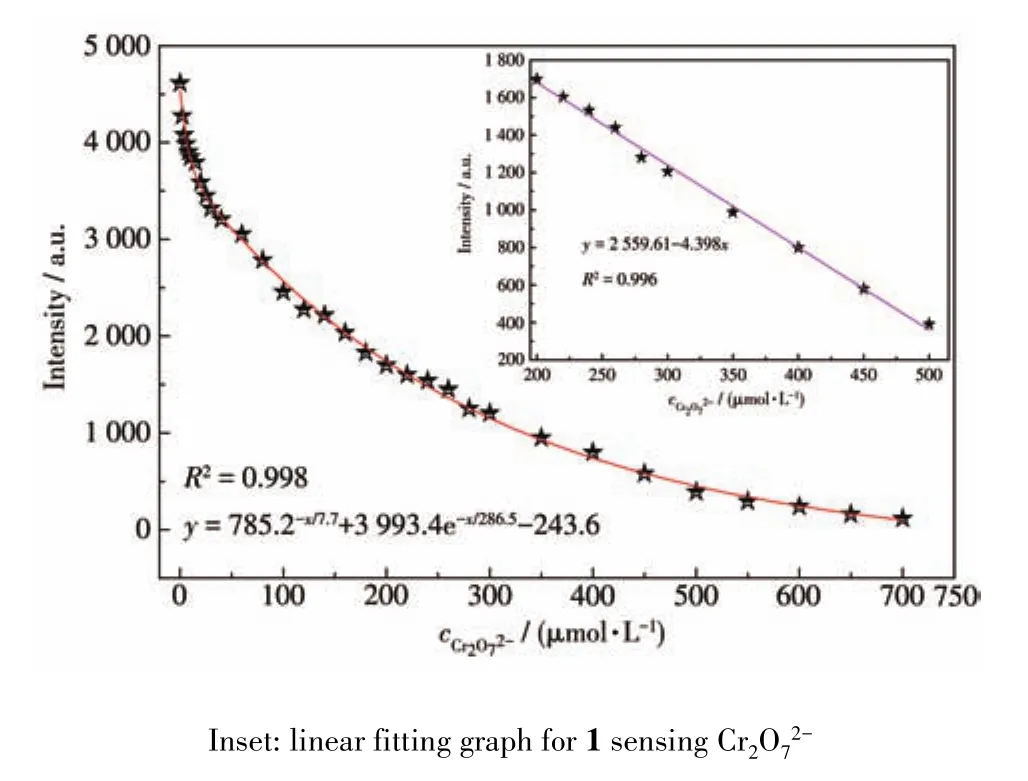
Fig.7 Nonlinear fitting graph for 1 sensing Cr2O72-
2.5 Electrochemical sensing of Tb(Na)-MOF
Catechol as an important phenolic compound is widely used in pharmaceutical industry and organic chemical industry,which has been identified as a high?ly toxic environmental pollutant because of its serious harm to the environment and organisms[20].Therefore,how to fastly detect catechol with low cost,high sensi?tivity,high reproducibility has been widely concerned.
As an excellent multifunctional material,MOFs have gradually attracted more and more attention in electrochemical fields such as capacitors and catalytic hydrolysis[21].However,there are no reports on the direct detection of catechol by electrochemical method.
The results of catechol detection of 1 by CV are shown in Fig.8.It can be seen from Fig.8 that the reduction current gradually increased with the increase of catechol concentration,and they were linearly related(Fig.8,inset):I=5.28×10-7-6.10×10-7c(R2=0.997 0).This indicates that Tb(Na)?MOF has a broad prospect in electrochemical sensing materials.
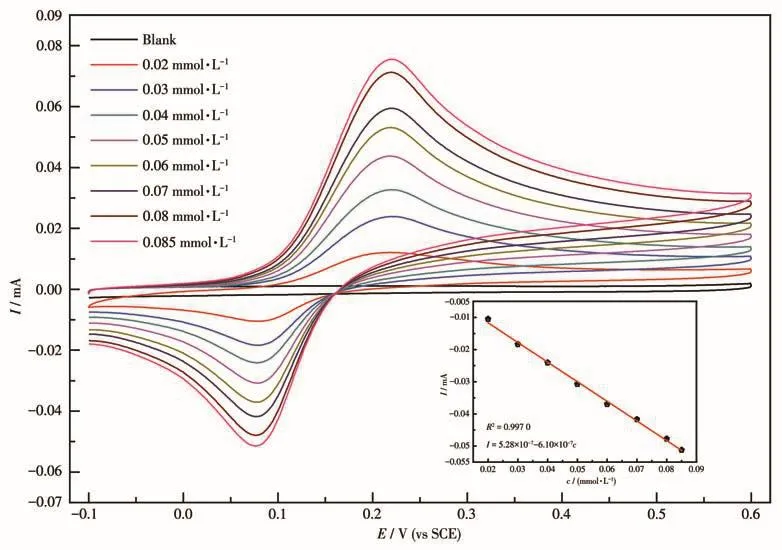
Fig.8 CV curves of catechol detection by 1/CPE
3 Conlusions
In summary,three novel luminescent 3D micropo?rous heterometallic MOFs(Ln(Na)?MOFs,Ln=Tb(1),Dy(2),Ho(3))were successfully constructed.Fluores?cence analysis revealed that Tb(Na)?MOF could act as a fluorescent probe for highly sensitive induced Fe3+and Cr2O72-(LOD of Cr2O72-:6.72 μmol·L-1)in aqueous solutions and could detect the environmental pollutants acetaldehyde molecules(LOD:0.125 μmol·L-1)effi?ciently and high sensitively.In addition,1/CPE for detection of catechol showed a good electrochemical activity.Finally,the multifunctional sensing properties of Tb(Na)?MOF may make it expected to be further developed as a potentially valuable detection material for environmental pollutants.
Conflicts of interest:The authors declare no competing financial interest.
Acknowledgments:This work was supported by National Natural Science Foundation of China(Grants No.22063010,21763028)and College Students Innovation and Entreprencur?ship Programme(Grants No.YCX2021101,D2020037).
Supporting information is available at http://www.wjhxxb.cn
 無(wú)機(jī)化學(xué)學(xué)報(bào)2021年11期
無(wú)機(jī)化學(xué)學(xué)報(bào)2021年11期
- 無(wú)機(jī)化學(xué)學(xué)報(bào)的其它文章
- Mn-Based Coordination Polymer:Facile Synthesis,Structure and Application in Glucose Electrochemical Sensing
- Preparation of Dehydrated Ni-Fe Hydrotalcite-like Compounds as an Eco-Friendly Catalyst for Highly Selective Acetalization of Biomass-Derived Furfural
- Synthesis,Structure Regulation and Characterization of Cadmium(Ⅱ)Complexes Based on Imidazole Carboxylic Acid Ligands
- Difunctional Effects of Organo-Modified T-Type Zeolite Membranes for Dewatering from Organic Solution
- Controlling Distribution of Gold Nanoparticles in Au@ZIF-8 Core-Shell Structures for Sensing Fluorescent Molecules with Photoluminescence
- Structures and Luminescence Property of Two Co(Ⅱ) and Cd(Ⅱ)Supramolecular Coordination Networks Created via Synergistic Effect of Coordination Bonds and Secondary Interactions
