Determination of Inorganic Anions and Melamine in Fertilizers by Ion Chromatography
Chen LIANG, Miaomiao ZHANG, Xiaojiao WANG, Yunli YAN, Pengfei FU
Qingdao Shenghan Chromatograph Technology Co., Ltd., Qingdao 266101, China
Abstract A chromatographic analysis method for determining inorganic anions and melamine in fertilizers was established using ion chromatography (IC). The fertilizer samples were extracted by ultrasonic method with 7 g/L trichloroacetic acid solution and centrifuged. The supernatant is purified by a solid phase extraction column. Then, the anions in the solution were purified using SH-AP-1 (250 mm×4.0 mm) as a separation column, and measured by a suppressed conductivity detector; the melamine in the solution was separated using SH-CC-4 (200 mm×4.0 mm) as a separation column and detected by a UV detector. The results show that the mass concentration of anions had a linear relationship with its peak area within a certain range, the linear correlation coefficient r of the standard curve was greater than 0.999, the recovery rate of spiked samples was 93.4%-104.4%, and the relative standard deviation (RSD) of the measured value (n=6) was less than 4%. Thus, this method is widely suitable for detection of anions and cations in a variety of fertilizer samples.
Key words Ion chromatography (IC), Fertilizer, Anion, Melamine
1 Introduction
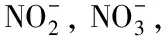
etc.
, achieve control of the production process and protection of the environment, ensure the normal and safe growth of crops, and maintain consumer health, it is necessary to establish a test method for determining anions and melamine in fertilizers. At present, there are many methods for determining anions and melamine in fertilizers such as ammonium thiocyanate titration method, spectrophotometric method, ion selective electrode method, ion chromatography combined with ICP-MS,etc.
Most of these methods are complex to operate, have a long test period, have interference, and are difficult to test for multiple ions. In addition, there are high-performance liquid method and gas-mass spectrometry in the standard NY/T 13722-2007 of the Ministry of Agriculture, as well as liquid chromatography-mass spectrometry. These methods have disadvantages such as the need for derivatization, complex operation, lengthy process, large volume of reagents, and low sensitivity. In this study, we used the extraction column purification, ion chromatography-conductivity method and ion chromatography-ultraviolet method to detect the content of anions and melamine in fertilizers. No derivatization is required. The operation is simple. Multiple ions can be tested in one injection. The method is practical and reliable, and can meet the needs of safety testing for fertilizer production and use.2 Materials and methods
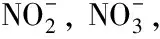
2.2 Instruments
CIC-D160 ion chromatograph, equipped with potassium hydroxide eluent generator, methanesulfonic acid eluent generator, SHD-6 constant temperature conductivity cell, and UC-3292 UV detector (Qingdao Shenghan Chromatograph Technology Co., Ltd., China); UPT-II-20L ultrapure water system (Sichuan ULUPURE Technology Co., Ltd., China); Mettler Tole-do AL-104 electronic balance (Mettler-Toledo Instruments Co., Ltd., Swiss); TG18-WS centrifuge (Changsha Wei’erkangxiangying Centrifugal Machines Co.,Ltd., China); KQ 2200 ultrasonic extractor (Kunshan Ultrasonic Instruments Co., Ltd., China).2.3 Methods
2.3.1
Chromatographic conditions. Conditions of ion chromatography-conductivity detector for anion detection: SH-AP-1 anion chromatography column (250 mm×4.0 mm) is a hydrophilic anion chromatography column; the eluent is a high-purity potassium hydroxide solution produced by the eluent generator; the gradient elution and concentration are shown in Table 1. The flow rate is 0.8 mL/min, the injection volume is 25 μL, the column temperature and the detector cell temperature are both 35 ℃, SHY-6 suppressor, the suppression current is 120 mA, and the system pressure is 10.2 MPa. Qualitative determination is taken by the retention time, and quantitative determination is achieved by the peak area. Conditions of ion chromatography-ultraviolet detector for detection of melamine: SH-CC-4 cation chromatography column (200 mm×4.0 mm), the eluent is the methanesulfonic acid solution produced by the methanesulfonic acid eluent generator, the eluent concentration is 5 mmol/L methanesulfonic acid solution (MSA), the flow rate is 1.0 mL/min, the system pressure is 7.7 MPa, the injection volume is 25 μL, the column temperature is 35 ℃, and the UV detector wavelength is 214 nm. Qualitative determination is taken by the retention time, and quantitative determination is achieved by the peak area.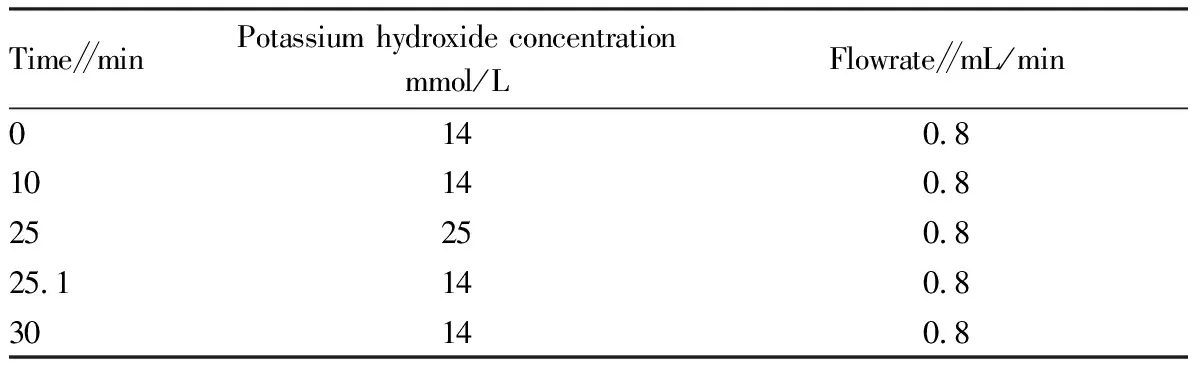
Table 1 Gradient elution procedure of mobile phase
2.3.2
Prepation of standard solution. 7 g/L trichloroacetic acid solution: weighed 7 g of trichloroacetic acid and diluted to 1 L with ultrapure water.2
.3
.3
Pretreatment of samples. Weighed 0.5 g of commercially available fertilizer samples that have been dried and crushed into a 100 mL volumetric flask, added 7 g/L trichloroacetic acid solution to a constant volume, shook up, and conducted ultrasonic treatment for 30 min. Centrifuged a part of the solution at 8 000 r/min for 10 min. Used a syringe to take the centrifuged supernatant and screened through a 0.22 μm aqueous syringe filter membrane. Then slowly pushed the sample solution into the activated Csolid phase extraction column and Ba solid phase extraction column at sampling speed less than 3 mL/min, after discarding the first 5 mL of the solution, performed the determination according to the working conditions of the instrument, and at the same time did a blank sample test.3 Results and analysis
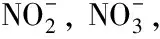
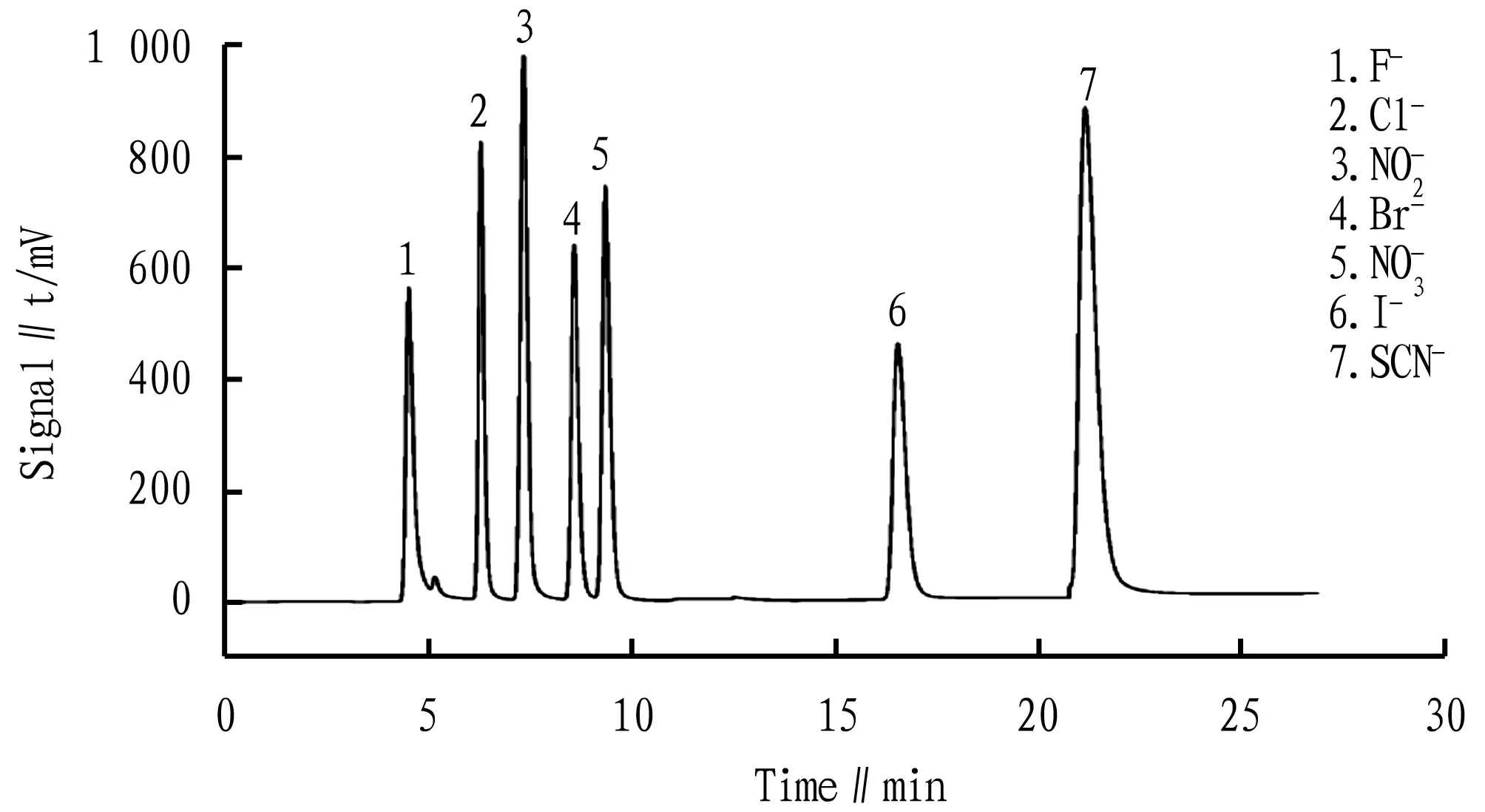
Fig.1 Standard product chromatogram for anions
Both SH-CC-3 and SH-CC-4 columns are weak acid cation columns. The SH-CC-3 matrix is a styrene-divinylbenzene polymer with a cross-linking degree of 55%, and the surface is grafted with carboxyl groups. SH-CC-4 chromatographic column is 7 μm styrene-divinylbenzene with 55% cross-linking degree, and weak acid functional groups are grafted on its surface to become polymer cationic chromatographic column packing. After testing, both chromatographic columns can test melamine. Through testing the melamine standard samples of the same concentration, it was found that the response value of the melamine standard product measured by SH-CC-4 was greater than that of SH-CC-3. In the actual sample, the content of melamine is very low, so we adopted the SH-CC-4 column. The standard chromatogram is shown in Fig.2.
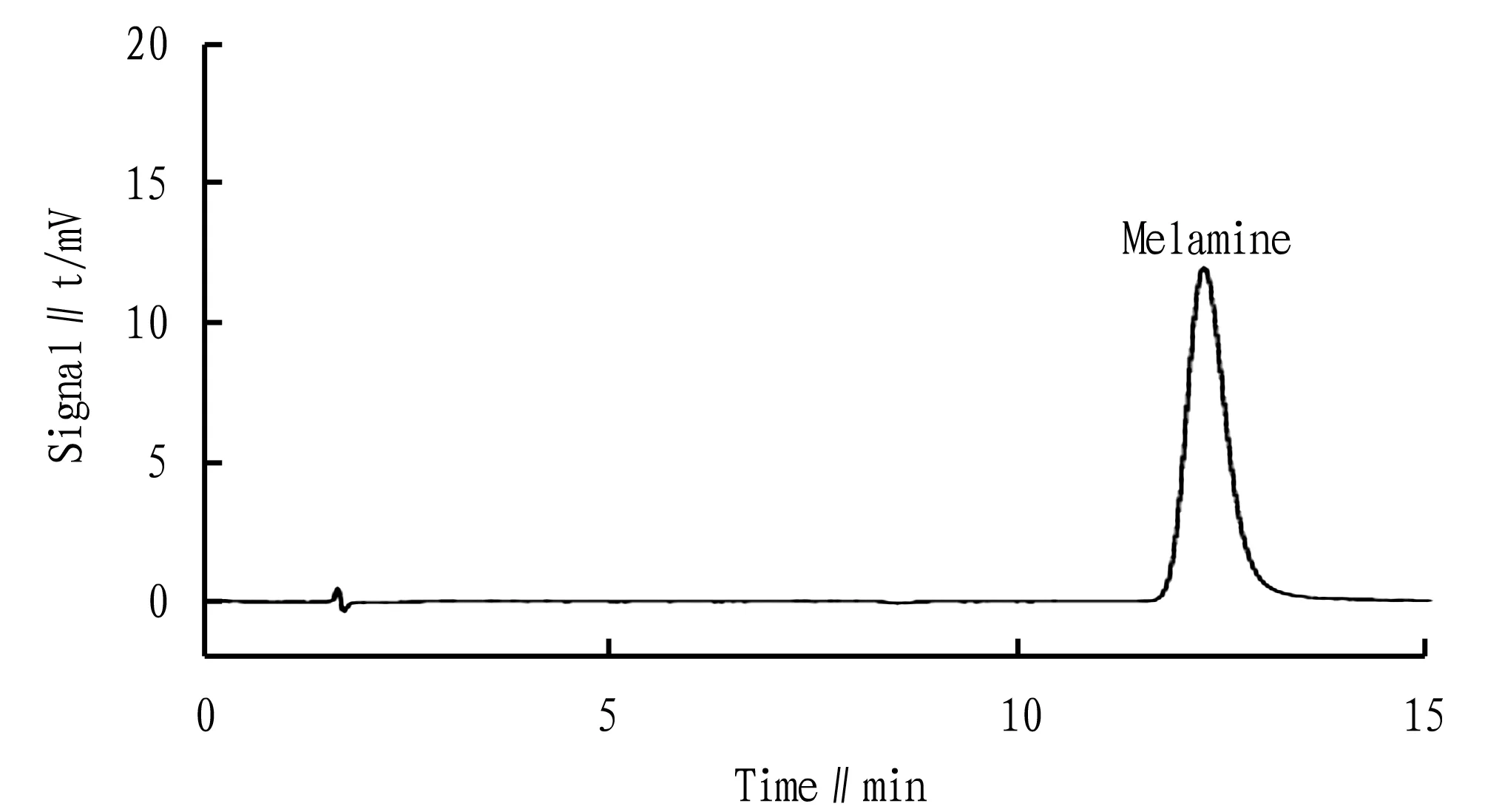
Fig.2 Standard product chromatogram for cations
3.2 Optimization of extraction methods
The anion pollutants to be tested in the fertilizer samples can be directly dissolved in water. Melamine is a small-molecule polar compound that is easily soluble in alkaline or acidic aqueous solutions and organic solvents such as methanol and acetonitrile. It has proved that melamine is more soluble in trichloroacetic acid solution, so we took trichloroacetic acid as the extractant. We detected the recovery rates of spiked samples of different concentrations of trichloroacetic acid, and the test results are shown in Table 2. From Table 2, it can be known that the recovery rate of melamine increases as the concentration of trichloroacetic acid increases. However, when the concentration of trichloroacetic acid was greater than 7 g/L, the recovery rate dropped instead, so we selected 7 g/L trichloroacetic acid as the extract.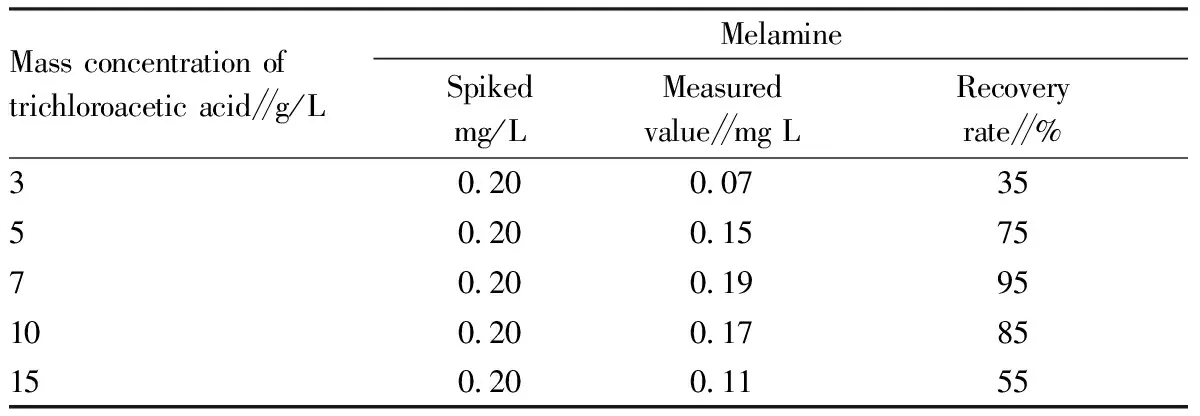
Table 2 Recovery rate of trichloroacetic acid as extractant melamine
3.3 Optimization of extraction method and extraction time
Most of the active components in fertilizers are water-soluble, and the pollutants to be tested are also soluble in water. Therefore, the extraction efficiency of water-soluble ions of pollutants in fertilizer samples is related to the extraction method used. In the experiment, we separately selected oscillation and ultrasonic extraction methods and then performed the ultrasonic extraction for 10, 20, 30, and 40 min to evaluate the effect on the extraction efficiency. The results show that the ultrasonic extraction had higher efficiency than the oscillation extraction, so we adopted the ultrasonic extraction. When the ultrasonic extraction was 20 min, the sample ions in the solution reached the maximum value. With the increase of the extraction time, the sample ions still increased, but the trend was weaker. Therefore, in order to speed up the pretreatment speed and save time, we adopted the ultrasonic extraction and set the ultrasonic time to 20 min.
3.4 Removal of organic matters
Organic-inorganic compound fertilizers and high-efficiency compound fertilizers have complex components, containing organic matter, pigments, protein and other hydrophobic substances, which affect separation, contaminate the chromatographic column and shorten the service life of the chromatographic column. Therefore, it is necessary to select a suitable pretreatment method to purify the sample before the test. We first screened sample through a 0.22 μm aqueous syringe filter membrane. Then slowly pushed the sample solution into the activated Csolid phase extraction column. After the sample passed the above-mentioned treatment process, the solution became clear obviously, indicating that the Csolid phase extraction column can effectively remove the organic matters in the sample.3.5 Removal of sulfate and phosphate
In the actual application process, some fertilizer samples are sulfate or phosphate fertilizers, such as potassium sulfate fertilizer, superphosphate, calcium magnesium phosphate fertilizer, phosphate rock powder,etc.
They contain a large amount of sulfate and phosphate, and can be dissolved in water, and produce high concentrations of sulfate and phosphate, which will affect the anion to be measured. At present, the most common method for removing high-concentration phosphoric acid and sulfuric acid is to use a Ba column. That is connecting a Ba column behind the Csolid phase extraction column. After the test, most of the sulfate and phosphate in the sample were removed. Although some were not removed, it would not affect the separation of the ions to be tested.3.6 Selection of detector
Melamine is a triazine nitrogen-containing heterocyclic organic amine compound. Its aqueous solution is weakly alkaline, with a pH of about 8. Under neutral or slightly alkaline conditions, it condenses with formaldehyde to form various methylol melamine molecules. If the test uses the suppression conductivity method to detect, the suppressed solution is neutral and cannot generate a signal in the conductivity detector. However, it exists in the form of cyanuric acid in slightly acidic solution (pH 5.5-6.5), so acidic eluent is needed. In this experiment, we selected methanesulfonic acid eluent, and used non-suppressive conductivity method and ultraviolet detection method to detect the samples. We found that the non-suppressive conductivity method is easily affected by the high concentration of cations in the sample, and the UV detection method has a lower detection limit and less interference, so we selected the UV detector. Through analyzing the standard solution of melamine with a UV detector, we found that the sensitivity of melamine was the highest at the wavelength of 214 nm. Thus, we selected 214 nm as the wavelength of the UV detector.
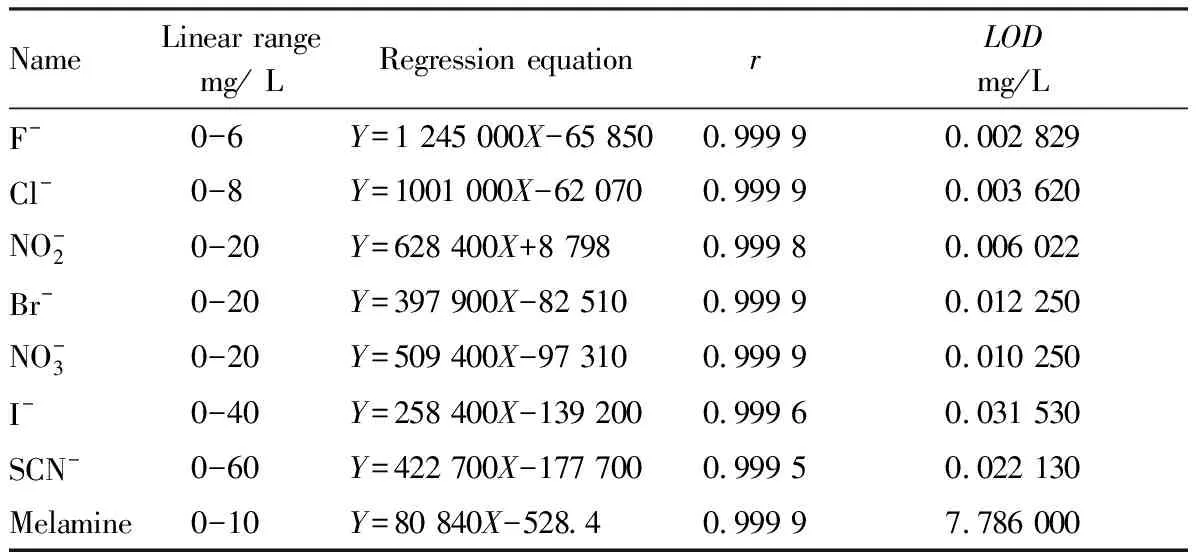
Table 3 Linear parameters, limit of quantification and limit of detection
3.8 Accuracy and precision of the method
We separately took samples of commercially available organic-inorganic compound fertilizer and nitrogen fertilizers, processed the fertilizer samples according to the test method, carried out the spike recovery test, tested in parallel 6 times, and calculated the relative standard deviation (RSD
) of recovery rate and measured value. The results are shown in Table 4. From Table 4, it can be known that the recovery rate was 93.4%-104.4%, and theRSD
is less than 4%, indicating that the method has good accuracy and precision.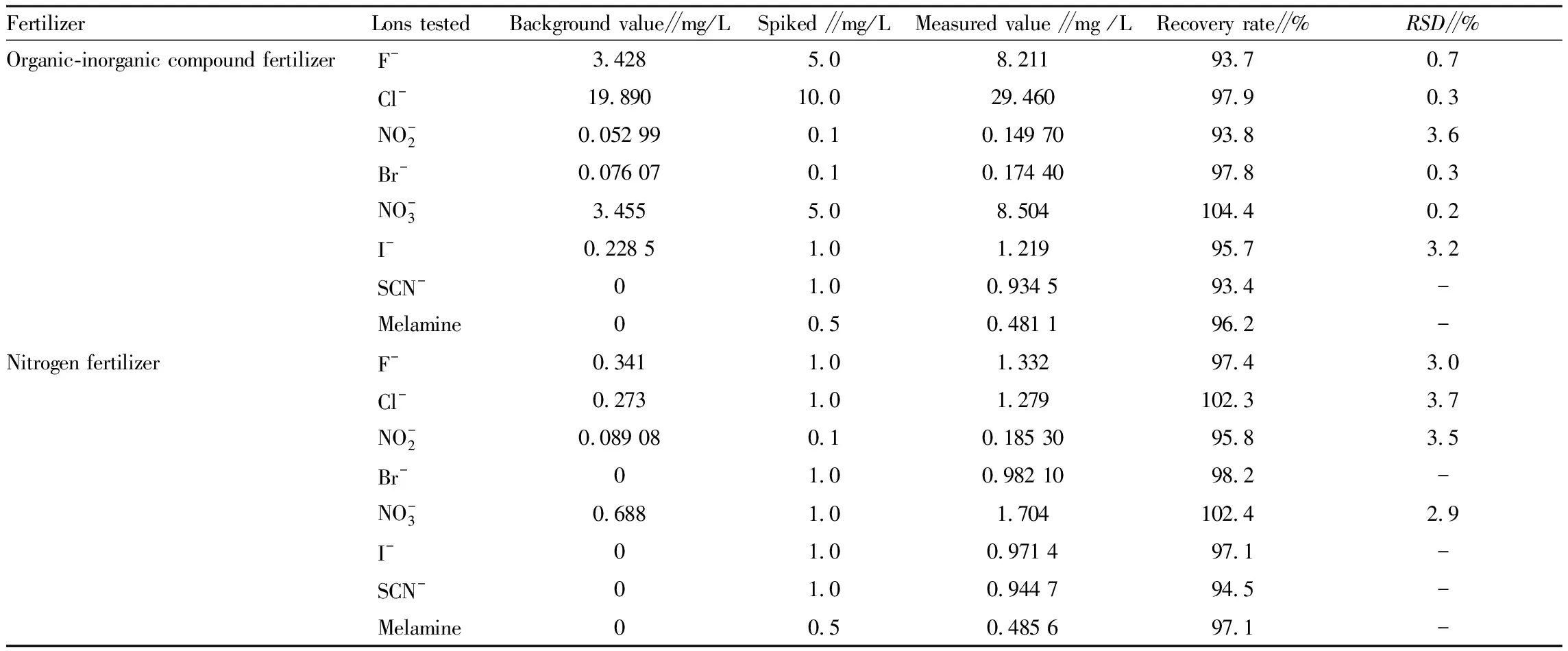
Table 4 Results of precision and recovery rate tests (n=6)
3.9 Determination of samples
Using the established method, we determined the target substances in a variety of commercially available fertilizer samples, and each sample was tested in parallel three times. The results are shown in Table 5. From Table 5, it can be seen that high-efficiency compound fertilizers and organic-inorganic compound fertilizers contain high levels of Fand Cl, so it is necessary to care about their impact on the soil and the environment during use.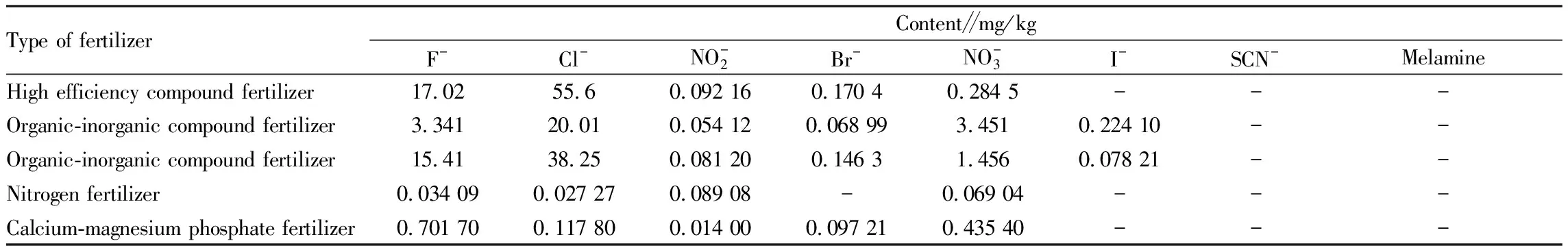
Table 5 Test of target ions in samples of commercially available fertilizers (n=3)
4 Conclusions
We established a method for rapid detection of anions and melamine in fertilizer samples by ion chromatography, and determined the anions and melamine content in fertilizer samples using this method. It can quickly determine the concentration of 7 anions and melamine, and has a good linear relationship with the peak area within a certain range, and has a high accuracy and precision. The processing before using the method is simple. In summary, the method is fast, has high sensitivity, wide application range, high precision, and can be widely used in the detection of anions and melamine in fertilizers. It is expected to play a guiding significance for fertilizer production and use process, and accordingly realizing the quality control of some ions in fertilizers.
 Asian Agricultural Research2021年8期
Asian Agricultural Research2021年8期
- Asian Agricultural Research的其它文章
- Attaching Importance to the Training of Young Researchers and Promoting the Echelon Construction of the Research Institute
- Research on Stimulating the County-wide Economic Vitality of Jingzhou City
- Farmers’ Ideological and Political Education under the Background of Rural Revitalization Strategy
- Study on Characteristics of Soil Seed Bank of Natural Grassland in Nierong County
- Evaluation on Terrain-Climate Superiority Degree at County Level in Yunnan Province
- Changes of China’s Position in the World Food Trade Network
