Adsorption of propylene carbonate on the LiMn2O4(100)surface investigated by DFT+U calculations?
Wei Hu(胡偉), Wenwei Luo(羅文崴),?, Hewen Wang(王鶴文), and Chuying Ouyang(歐陽楚英)
1Department of Physics,Laboratory of Computational Materials Physics,Jiangxi Normal University,Nanchang 330022,China
2College of Chemistry and Chemical Engineering,Hubei Key Laboratory for Processing and Application of Catalytic Materials,Huanggang Normal University,Huanggang 438000,China
Keywords: Li-ions batteries,electrolyte,density functional theory,surface,propylene carbonate
1. Introduction
Li-ion batteries have received significant attention due to their high energy density and environmental friendliness,and are widely used as energy storage devices of small electronic products and electric vehicles. Currently, the research and development of Li-ion batteries mainly focus on improving the performance of existing battery systems and developing new battery materials to achieve specific applications.[1,2]In the Li-ion batteries system, the cathode material, as a provider of Li-ions, plays a key role in the electrochemical performance of the battery,[3-5]regarding safety,price,capacity,etc.Different types of cathode materials have been considered for Li-ion batteries, including layered structured materials LiMO2(M=Co,Ni,Mn),[6-11]spinel structured material LiMn2O4,[12-15]Li-rich material Li2MnO3,[16-18]and olivine structured material LiFePO4.[19-22]Among these materials,spinel LiMn2O4has attracted much attention because of the following advantages: (i)manganese is low-priced,abundant;(ii) LiMn2O4shows high working voltage with a theoretical capacity of 148 mAh/g;(iii)LiMn2O4has a three-dimensional channel for the reversible diffusion of Li+ions.[23,24]
However,LiMn2O4also shows some undesirable features as a positive electrode,such as disproportionated reaction and Jahn-Teller distortion of Mn3+ion.[14,25,26]Doping and surface coating are considered to be effective methods to improve the battery performance. Zhang et al.[25]found that Al/Co dual-doped LiMn2O4showed a good cycling stability.Ouyang et al.[15]pointed out that Al2O3-covered LiMn2O4(100)surface inhibited the formation of Mn3+ions. Although some progress has been achieved in the investigation of LiMn2O4,there are still problems to be solved in order to further improve the electrochemical performance of LiMn2O4,[27-29]such as severe capacity degradation at high temperatures, interfacial reaction,etc.
In the present work, we studied the interfacial reaction of propylene carbonate C4H6O3(PC) on the LiMn2O4(100)surface and proposed the corresponding reaction mechanism with the help of density functional theory(DFT)calculations.The results provide a theoretical explanation to the better cycling performance of PC based electrolyte for the LiMn2O4cathodes.
2. Computational details
All calculations of interfacial reaction were performed using the projector augmented wave (PAW) method based on DFT+U calculations as implemented in the Vienna ab initio simulation package (VASP) code.[30-32]The exchangecorrelation functional was modeled using the Perdew, Burke,and Ernzerhof (PBE) within the spin-polarized generalized gradient approximation (GGA). The plane wave cut-off energy of 520 eV and k sampling with 3×3×1 k-point mesh in Brillouin zone were adopted for all surface adsorption models. Li-2s1,C-2s22p2,H-1s1,Mn-3d64s1,and O-2s22p4were modeled as valence electrons. The Hubbard U correction term was used to improve the description of the localized Mn-3d electrons.[33]The on-site U parameter for the Mn-3d electrons was set to be 4.5 eV based on previous study.[34]
In order to characterize the adsorption strength of PC molecule on LiMn2O4(100) surface, we calculated the adsorption energy(Eads)at different adsorption sites with different orientations on the molecule. The adsorption energy was calculated according to the following equation:
Eads=Etotal?Eslab?EPC,
where Etotalrepresents the total energy of the slab after adsorbing molecules,Eslaband EPCare the energies of the clean slab and the isolated PC molecule, respectively. A negative value of Eadscorresponds to an exothermic and favorable absorption process. The larger the negative value is, the more stable the adsorption is.
3. Results and discussion
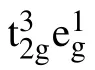
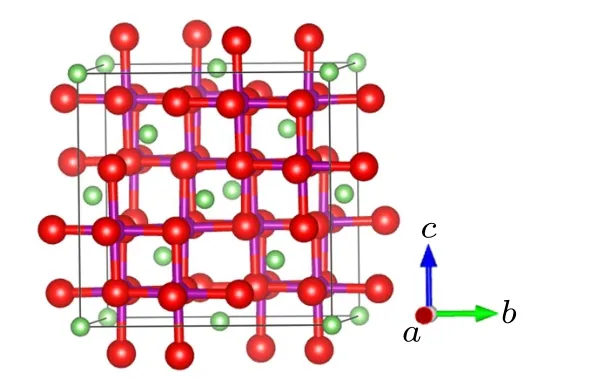
Fig.1. The structural model of the spinel LiMn2O4. The green,purple,and red balls are Li,Mn,and O atoms,respectively.
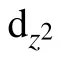
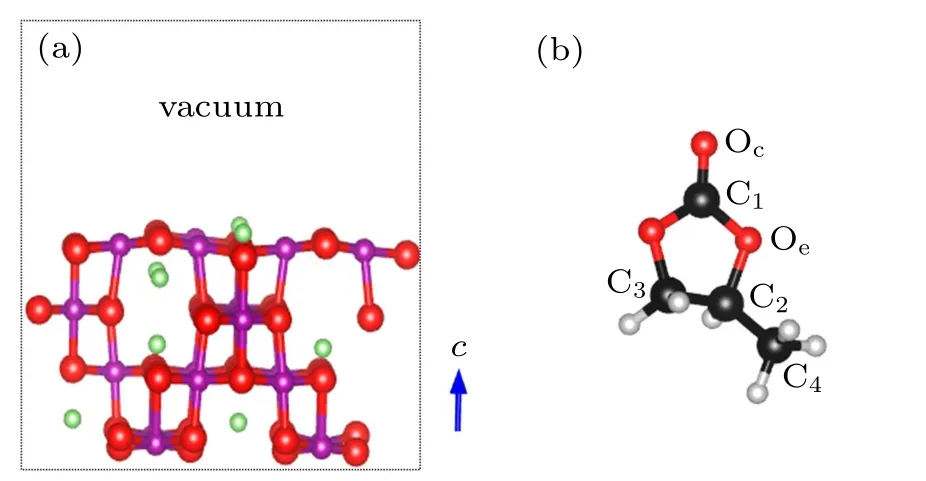
Fig.2. Structural models of(a)LiMn2O4 (100)surface and(b)propylene carbonate C4H6O3 (PC)molecule. The green,purple,black,gray,and red balls are Li, Mn, C, H, and O atoms, respectively. Oc and Oe represent the carbonyl and ethereal oxygens,respectively. C1-C4 is the code for the C atom.
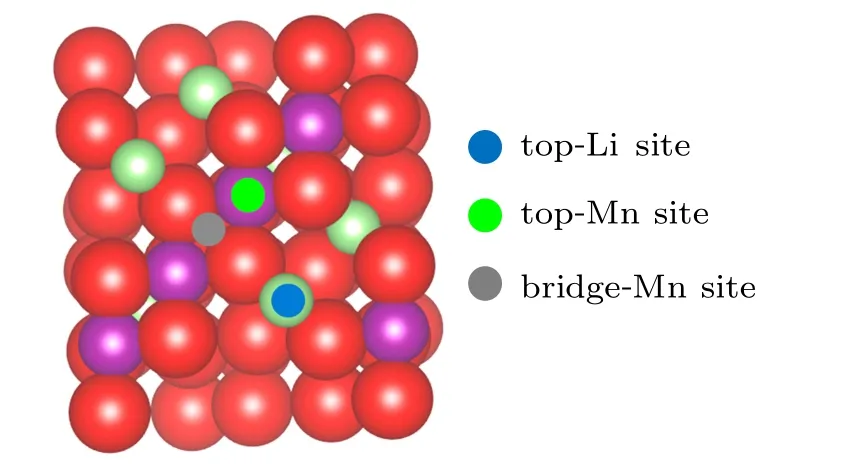
Fig.3. Schematic diagram of adsorption sites on LiMn2O4 (100)surface. The green,purple,and red balls are Li,Mn,and O atoms,respectively.
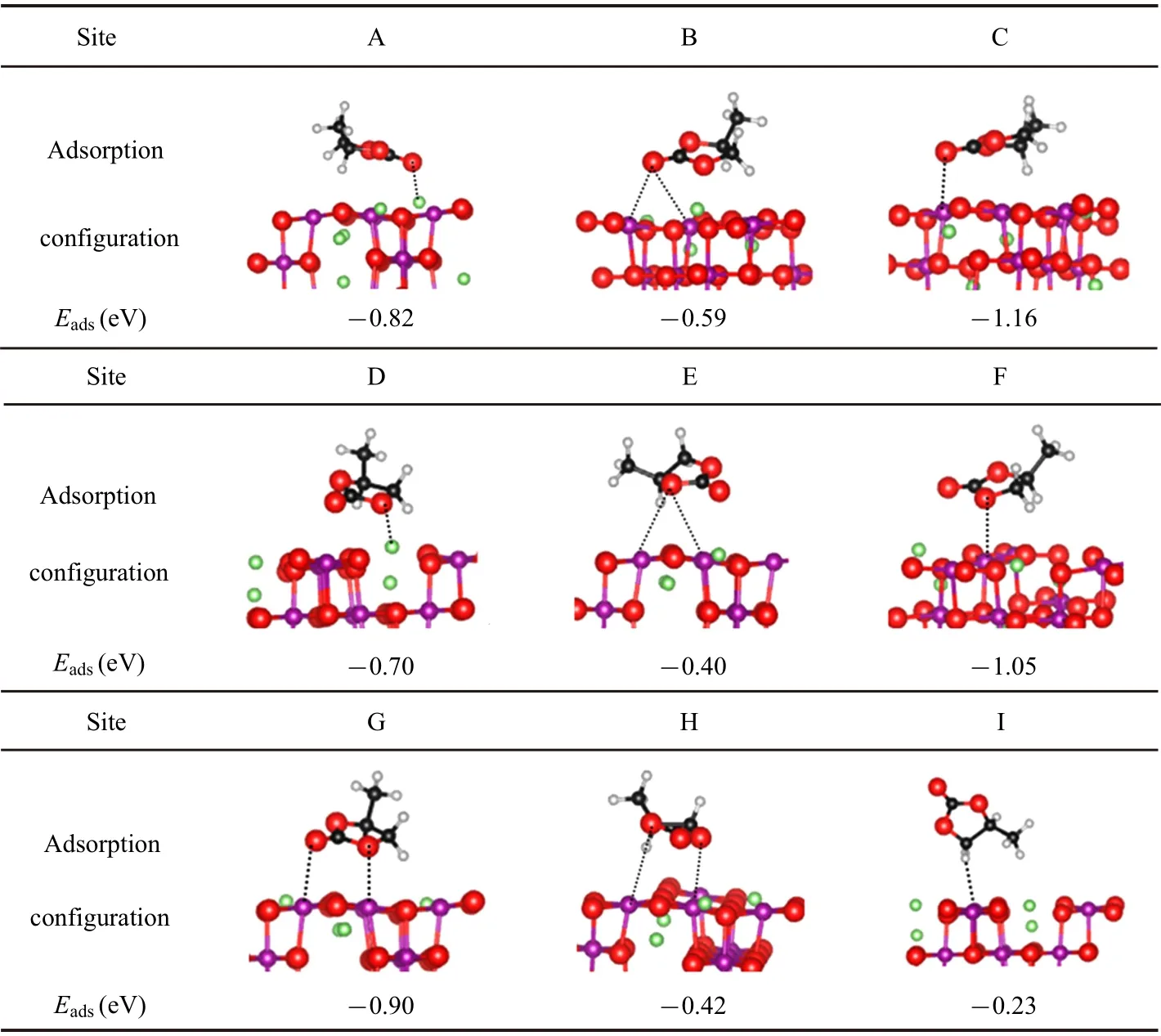
Table 1. Various adsorption configurations and corresponding adsorption energy Eads of PC adsorbed on LiMn2O4(100)surface. The green,purple,black,gray,and red balls are Li,Mn,C,H,and O atoms,respectively.
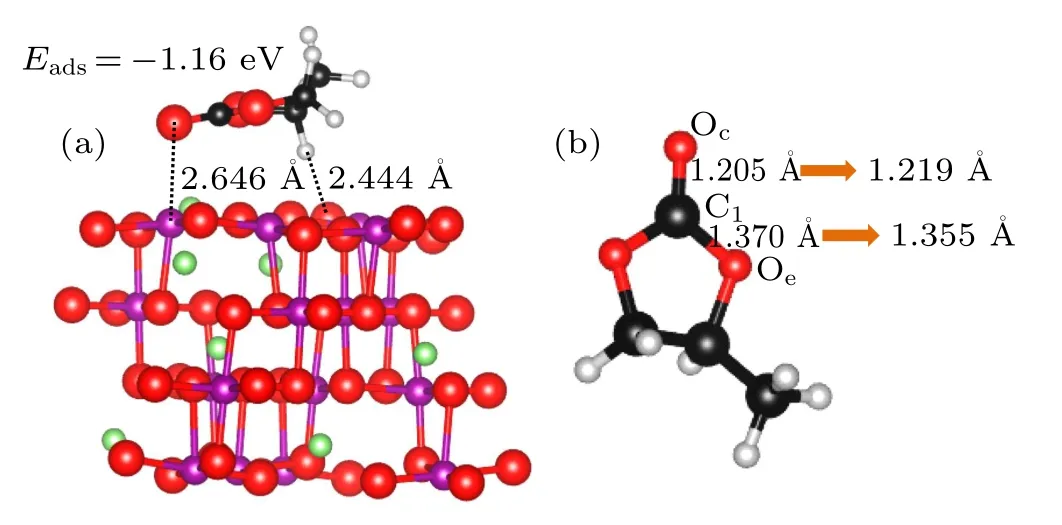
Fig.4. Most stable adsorption configurations for the PC molecule on the LiMn2O4 (100) surface (a) and structural changes of PC molecule after adsorption(b). The green,purple,black,gray,and red balls are Li,Mn,C,H,and O atoms,respectively.

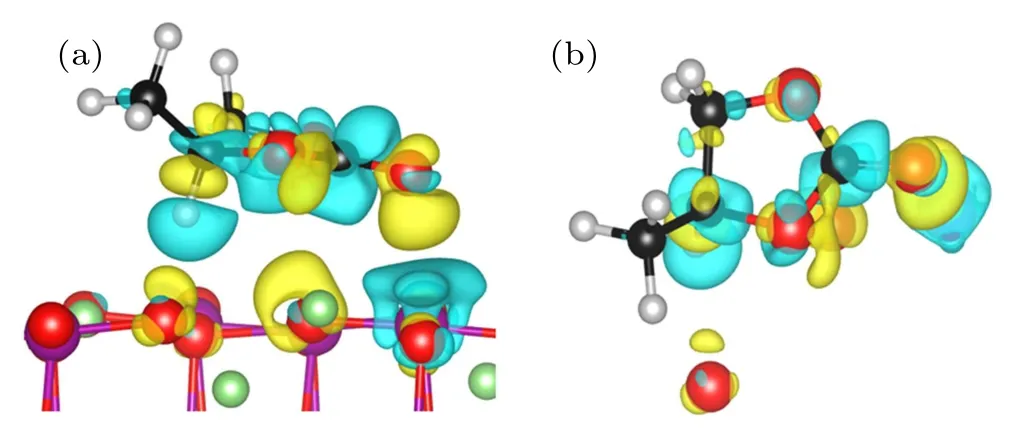
Fig.5. Side view(a)and top view(b)of the differential charge density ρdiff =ρtotal ?ρsurface ?ρPC plotof PC molecule adsorbed on LiMn2O4 (100)surface. The yellow regions indicate increased electron density,and blue regions represent decreased electron density.

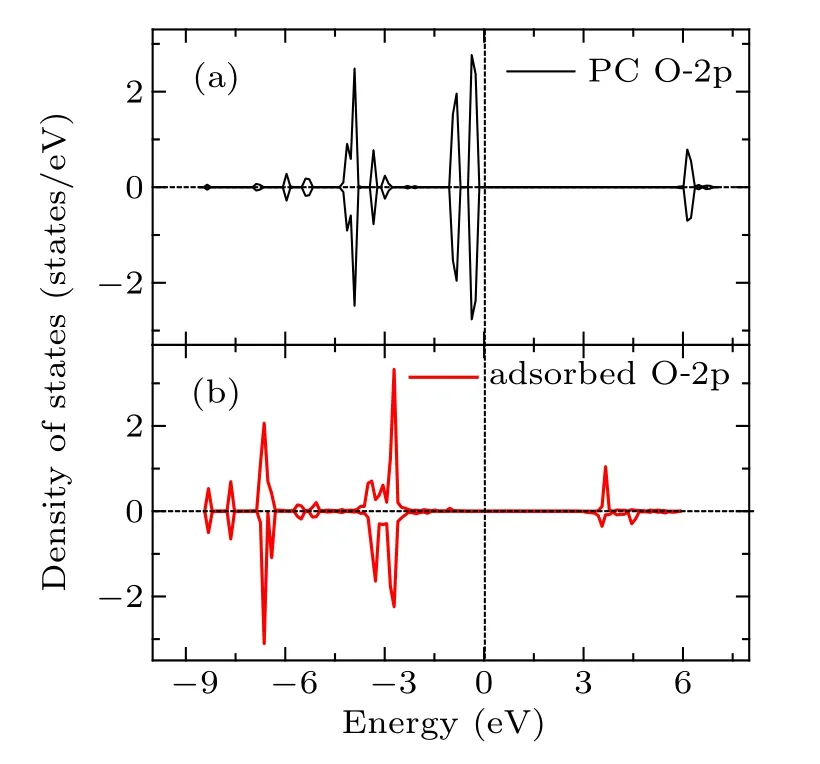
Fig.6. The PDOS for the 2p orbital of Oc atom of PC molecule before and after adsorption.
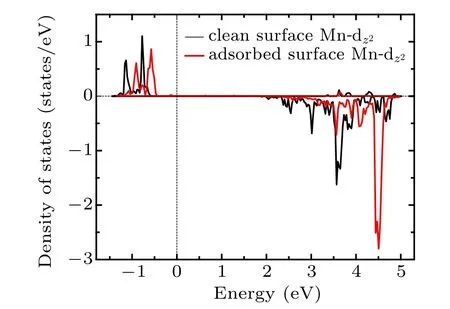
Fig.7. The PDOS for the component of the eg orbital of Mn atom which interacts with Oc before and after adsorption.
4. Conclusions
In summary, various adsorption configurations of the propylene carbonate (PC) molecule on LiMn2O4(100) surface have been studied base on spin-polarized DFT+U calculations. The results show that the PC molecule prefers to interact with Mn atom in the LiMn2O4(100)surface via the carbonyl oxygen (Oc), with the adsorption energy of ?1.16 eV,which is an exothermic reaction. There is obvious electron transfer between adsorbed molecules and the surface during the adsorption of molecules. The dz2component of the egorbital of surface Mn3+ion is the donor of electrons, while the 2p orbital of Ocis the acceptor of electrons, resulting in the stabilizing of the Mn3+ions in the LiMn2O4(100) surface.Our results suggest that increasing Mn coordination with O atoms at the surface by coating or molecule adsorption (such as carbonyl ad-layer, hydroxyl ad-layer, metallic oxide) can effectively inhibit the reactivity of Mn3+,thereby enhance the surface stability of the Mn-based cathode material.
- Chinese Physics B的其它文章
- Nonlocal advantage of quantum coherence in a dephasing channel with memory?
- New DDSCR structure with high holding voltage for robust ESD applications?
- Nonlinear photoncurrent in transition metal dichalcogenide with warping term under illuminating of light?
- Modeling and analysis of car-following behavior considering backward-looking effect?
- DFT study of solvation of Li+/Na+in fluoroethylene carbonate/vinylene carbonate/ethylene sulfite solvents for lithium/sodium-based battery?
- Multi-layer structures including zigzag sculptured thin films for corrosion protection of AISI 304 stainless steel?

