New guidelines for the diagnosis and management of pulmonary embolism: Key changes
Anastasia Erythropoulou-Kaltsidou, Stelina Alkagiet, Konstantinos Tziomalos
Abstract
Key words: Pulmonary embolism; Guidelines; Diagnosis; Treatment; D-dimers;Pregnancy
INTRODUCTION
Venous thromboembolism (VTE), which includes both deep vein thrombosis (DVT)and pulmonary embolism (PE), is an important public health problem. The annual incidence of first-time VTE in the United States is 71-117 cases per 100000[1]. Moreover,the 28-d case fatality rate after a first episode of VTE is approximately 11%[2].However, PE-related mortality rates have declined recently[3,4]. This decrease could be due to improvements in the diagnosis and managements of PE[4]. However, the decrease in PE-mortality might also be related to the overdiagnosis of PE, due to introduction and overuse of computed tomographic pulmonary angiography, in which non-clinically important PE is diagnosed and treated[5].
In August 2019, the European Society of Cardiology (ESC) in collaboration with the European Respiratory Society released the new guidelines for the diagnosis and management of PE[6]. This editorial will focus on the basic changes between the recent guidelines of the ESC for the diagnosis and management of PE and the previous guidelines that were published in 2014.
CHANGES IN THE DIAGNOSIS OF PULMONARY EMBOLISM
Flow chart for the diagnosis of pulmonary embolism was shown in Figure 1. Based on the new ESC guidelines, instead of a fixed-cut off level of D-dimers (500 ng/mL), an age-adjusted cut-off level of D-dimers should be considered to exclude PE in patients with low or intermediate clinical possibility for PE and in those where PE is unlikely[7,8]. The age-adjusted cut-off level of D-dimers is calculated by multiplying the age of the patient by 10 (for patients older than 50 years). Thus, in a 60-year-old patient who has a low or intermediate clinical possibility for PE or who is unlikely to have PE, D-dimers levels < 600 ng/mL (i.e., age × 10) instead of D-dimers levels < 500 ng/mL (i.e., the fixed-cut off level) excludes PE. On the other hand, in a 40-year-old patient who has a low or intermediate clinical possibility for PE or who is unlikely to have PE, the fixed-cut off D-dimers level of < 500 ng/mL should be used, since the patient is younger than 50 years.
A D-dimer test adapted to clinical possibility should also be considered instead of fixed cut-off level of D-dimer[6]. Based on the YEARS study, if D-dimer levels are <1000 ng/mL and none of the 3 clinical items of Wells score (signs of DVT, hemoptysis or PE being the most likely diagnosis) are present or if D-dimer levels are < 500 ng/mL and one or more clinical items of Wells score are present, then a diagnosis of PE should be excluded[9].
There is also a change in the class of recommendation for the use of D-dimer levels during pregnancy and the post-partum period. According to the new guidelines, Ddimer measurement and clinical prediction rules should be considered to exclude PE during pregnancy and post-partum period[6]. Moreover, in case of suspected PE during pregnancy or the first 6 weeks post-partum, a specific diagnostic workup is provided to rule out or confirm the diagnosis of PE[6]. This updated diagnostic algorithm is based on recently published multicenter trials[10,11].
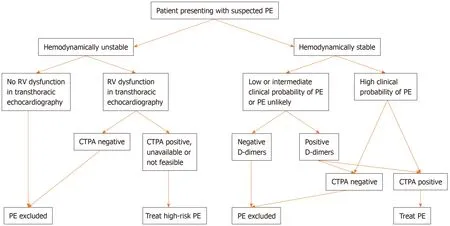
Figure 1 Flow chart for the diagnosis of pulmonary embolism. PE: Pulmonary embolism; RV: Right ventricle; CTPA: Computed tomography pulmonary angiography.
Furthermore, the 2019 guidelines summarize not only the advantages and disadvantages of the various diagnostic imaging tests but also describe and compare the exposure to radiation with the different tests.
Another change refers to the use of lower limb compression ultrasonography(CUS). The previous guidelines mentioned that, if CUS reveals proximal DVT in a patient and there is clinical suspicion of PE, a diagnosis of PE is established. However,the new recommendation in the 2019 guidelines, is that, if a positive CUS is used for the confirmation of PE, then risk assessment for PE severity and early mortality should be consider to guide further management[6].
In the 2019 guidelines, the role of ventilation/perfusion SPECT in the diagnosis of PE is emphasized more compared with the 2014 guidelines. In the new guidelines, it is mentioned that ventilation/perfusion SPECT may be considered for the diagnosis of PE[6]. However, more studies are needed to define the best SPECT technique.
CHANGES IN RISK ASSESSMENT OF PULMONARY EMBOLISM
In the 2019 guidelines, there is a definition of haemodynamic instability, which indicates acute high-risk PE. Three clinical manifestations of haemodynamic instability are mentioned (cardiac arrest, obstructive shock and persistent hypotension) and for each one, a clear definition is given, so that clinicians can decide if the patient is hemodynamically unstable or not. More specifically, haemodynamic instability is defined as: (1) Cardiac arresti.e., need for cardiopulmonary resuscitation;(2) Obstructive shocki.e., systolic blood pressure (SBP) < 90 mmHg (or need for vasopressors to achieve SBP ≥ 90 mmHg) despite adequate filling status and endorgan hypoperfusion (altered mental status, cold/clammy skin, oliguria/anuria or increased serum lactate); or (3) Persistent hypotensioni.e. SBP < 90 mmHg or SBP drop ≥ 40 mmHg, lasting > 15 min and not caused by new-onset arrhythmia,hypovolemia or sepsis.
Although the first risk stratification is based on the clinical manifestations of haemodynamic instability, assessment of PE severity and PE-related, early mortality risk is also recommended for patients with PE but without symptoms and signs of haemodynamic instability[6]. The prognostic criteria, on which the further risk stratification is based, are separated into 2 categories: (1) Clinical, imaging and laboratory parameters, the most important of which is right ventricular dysfunction;and (2) Comorbidities and other conditions that have an adverse effect on early prognosis.
In the 2019 guidelines, emphasis is given to right ventricular dysfunction, which is associated with increased risk for short-term mortality in hemodynamically stable patients with PE. Right ventricular dysfunction should be evaluated either with ultrasound or with laboratory prognostic biomarkers [cardiac troponins, brain natriuretic peptide (BNP) or proBNP], even if the Pulmonary Embolism Severity Index (PESI) is low or the simplified PESI (sPESI) is zero[6,12,13].
For further risk stratification of the severity of PE in patients without hemodynamic instability, use of validated scores (the Bova and the H-FABP scores) that combine clinical, imaging and laboratory PE-related prognostic factors might also be considered[6,14,15].
CHANGES IN THE TREATMENT OF PULMONARY EMBOLISM
Patients with PE are treated according to their hemodynamic status and their risk profile. More specifically, thrombolysis is recommended in patients with PE who are hemodynamically unstable and at high risk. If thrombolysis is contraindicated or unsuccessful, surgical pulmonary embolectomy or percutaneous catheter-directed therapy might be considered[6,16,17]. Even though reperfusion therapy might be lifesaving, it is not indicated in all patients with PE because of the increased bleeding risk[6,16,18].
The new guidelines also mention the possibility of early discharge (i.e., at 24 h) in patients without severe comorbidities, who are not of high risk for sudden death and in whom proper medical management at home and proper medical follow up can be ensured[6]. This recommendation is based on the results of the multi-center HESTIA trial, which evaluated the out-of-hospital treatment in patients with low-risk PE and showed that they could safely be treated at home. In fact, only 2% of these patients experienced recurrent VTE and none of these episodes occurred during the first 7 d of treatment[19]. In another study, patients with PE and a low PESI score were treated at home and had a very low PE-related and all-cause mortality[20]. The new guidelines also suggest that proBNP levels, right ventricular function and the presence of thrombus in the right heart could be useful for guiding the decision of early discharge[6,21].
Long-term treatment of patients with PE includes anticoagulant therapy for at least 3-6 mo[6]. Whether the treatment should be extended beyond this period depends on the risk of recurrence[6]. In patients with PE due to a treatable or transient risk factor,discontinuation of anticoagulation at 3 mo is recommended[6].
Direct-acting oral anticoagulants are the treatment of choice in patients with PE,except during pregnancy and in patients with severe renal impairment or the antiphospholipid syndrome[6]. In patients with antiphospholipid syndrome, vitamin K antagonists indefinitely are the treatment of choice[6]. In pregnant women and in patients with severe renal impairment, low-molecular weight heparin is the recommended treatment[6]. Patients with cancer should also be treated with lowmolecular weight heparin even though Direct-acting oral anticoagulants can also be considered based on the results of recent trials[22,23].
The use of vena cava filters is suggested only in patients with absolute contraindications to anticoagulant treatment[6]. However, they do not appear to reduce the risk of PE recurrence or PE-related mortality[24,25].
Finally, all patients with PE should be followed-up regularly because of the increased incidence of cancer (which might not be detectable at the time of PE), the risk of bleeding complications and the risk for development of chronic thromboembolic pulmonary hypertension[6].
CONCLUSION
The recent guidelines for the diagnosis and treatment of PE include several key changes which facilitate the management of this common and potentially lifethreatening medical emergency (Table 1). Knowledge and adherence to these guidelines will improve the outcome of these patients.
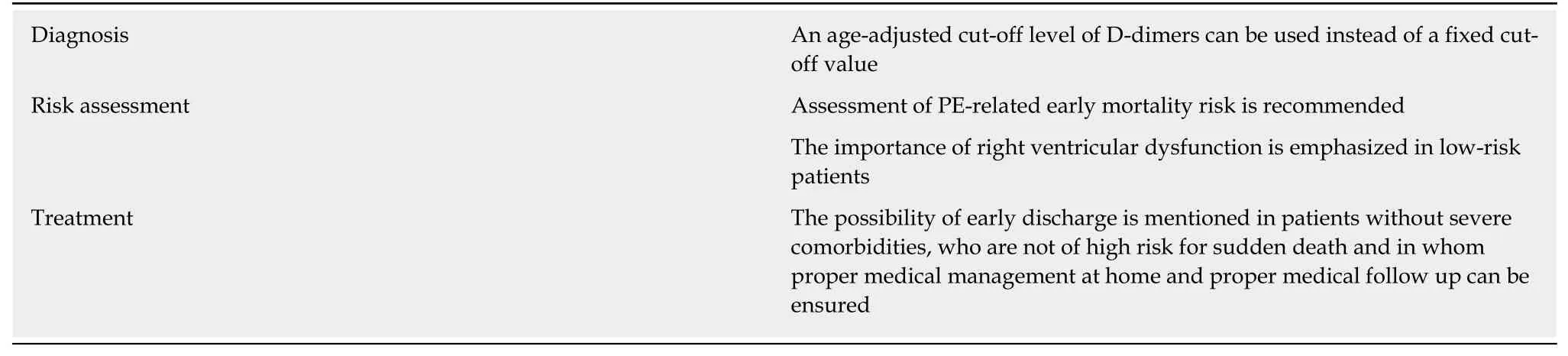
Table 1 Key changes in the 2019 guidelines of the European Society of Cardiology regarding the diagnosis and treatment of pulmonary embolism
 World Journal of Cardiology2020年5期
World Journal of Cardiology2020年5期
- World Journal of Cardiology的其它文章
- Management of hypertension in COVID-19
- Incidental discovery of right ventricular lipoma in a young female aImaging investigations and diagnosissociated with ventricular hyperexcitability: An imaging multimodality approach
- Preoperative nuclear stress testing in the very old patient population
- Access to smart devices and utilization of online health resources among older cardiac rehabilitation participants
- Nicotine-induced adrenal beta-arrestin1 upregulation mediates tobacco-related hyperaldosteronism leading to cardiac dysfunction
- Management of adults with coarctation of aorta
