Fabrication of Cu/rGO/MoS2 nanohybrid with energetic visible-light response for degradation of rhodamine B
Huanjing Liang,Peng Hua,Yufei Zhou,Zhimin Fu,Jiaoning Tang,Junfeng Niu,*
a Shenzhen Key of Special Functional Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, China
b Research Center for Eco-Environmental Engineering, Dongguan University of Technology, Dongguan 523808, China
c Ministry of Education Key Laboratory of Ecology and Resource Use of the Mongolian Plateau, School of Ecology and Environment, Inner Mongolia University,Hohhot 010021, China
Keywords:
CRM
Ternary nanostructures
Photodegradation
Rhodamine B
ABSTRACT
A novel visible-light-driven Cu/rGO/MoS2 (CRM) ternary nanostructure as a photocatalyst with high catalytic activity towards environmental purification using solar energy was successfully synthesized through a facile solvothermal method.It was found that the nanoflower structure of MoS2 increased the number of its exposed edges.Meanwhile rGO as a catalytic substrate played a role of charge-carrier channel to improve the separation of holes and electrons,which originated from the band gap absorption of MoS2.The content of Cu in photocatalyst affected photocatalytic performance obviously.And the optimal 30%Cu/rGO/MoS2 possessed the highest photocatalytic performance,which could be attributed to the improved separation of charges and synergistic effects among Cu, rGO and MoS2.The removal efficiency of Rhodamine B(RhB)over CRM was up to 100%in 5 min.CRM as a photocatalyst maintained good reproducibility and stability during 3 times of the recycle experiments.These results indicate CRM is a promising photocatalyst for degrading organic pollutants in wastewater.
In recent years, environmental pollution has been an increasingly serious issue due to the imperfect prevention and the lake of anticipation of the negative impact resulting from the rapid development.Water pollution caused by industrial synthetic dyes and pigments has seriously disturbed the water ecosystem,wastewater treatment technology has attracted widespread attention.Nowadays, there are many technologies to remove pollutants in wastewater, such as flocculation and sedimentation,microbial decomposition, molecular sieve adsorption, photocatalytic degradation.While among these technologies, photocatalytic degradation is known as a promising technology, which may consume only solar energy to mineralize organic pollutants in water or wastewater [1-12].
Among reported photocatalysts,the transition-metal dichalcogenides (TMDs) exhibit wide response spectra and potential in environmental contamination control [13-15].Especially, molybdenum disulfide(MoS2),a typical TMD,exhibits not only a narrow band gap but also two-dimensional layered graphene-like structures [16-19].These properties result in strong absorption of visible light and smooth pathway for charge transfer.However,there are two matters of concern over MoS2, which inhibits its catalytic performance to a certain extent.Firstly, the agglomeration of MoS2caused by the Van der Waals bond between MoS2layers decreases the number of active sites that lead to a lower catalytic efficiency.For the other thing,the poor intrinsic electrical conductivity of MoS2impedes charge transport and result in lower effective separation of charges [20-23].Taking these factors into consideration, a high efficiency photocatalyst of functional MoS2with more active sites and higher electrical conductivity via a facile method should be designed.Reduced graphene oxide (rGO) has higher electron mobility and conductivity,which is widely used in semiconductor modification to improve the separation and migration of its carriers to achieve higher electrocatalytic or photoelectrocatalytic properties[24-26].Moreover,graphene is a two-dimensional material with a large surface area,which is very suitable as a catalytic substrate for MoS2[27-29].Cu-based materials have recently attracted extensive attention in photocatalytic applications due to its abundant natural reserves,low cost and good conductivity [30-32].The functioned MoS2with combination of Cu and rGO is an effective method to improve the conductivity and inhibit the agglomeration of MoS2.
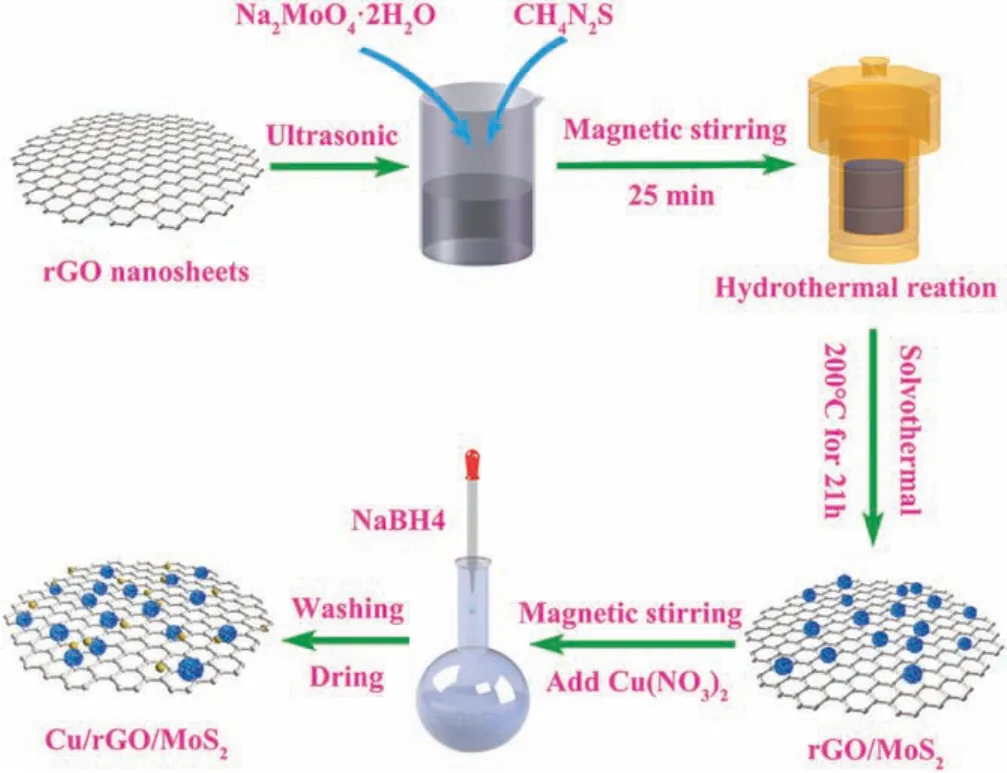
Scheme 1.Schematic diagram of the Cu/rGO/MoS2 preparation.
As outlined in Scheme 1, a solvothermal method was used to synthesis a novel visible-light-driven Cu/rGO/MoS2ternary nanostructure.The morphology and structure of the materials were characterized by SEM/TEM images (Fig.S1 in Supporting information), Raman spectra (Fig.S2 in Supporting information)and nitrogen sorption isotherm(Fig.S3 and Table S1 in Supporting information).The crystal structure was detected by XRD (Fig.1).For the prepared MoS2sample, the diffraction peaks at 13.97°,33.09°, 39.11°, 58.82°and 68.8°correspond to (002), (100), (103),(110) and (201) facets of MoS2(JCPDS card No.37-1492),respectively.Due to the weak crystallization of rGO, rGO/MoS2displayed similar XRD pattern to MoS2[33,34].Furthermore, the apparent diffraction peaks of Cu were observed in XRD patterns of the CRM hybrid.The diffraction peak located at 46.13°and 48.59°were assigned to(111)and(200)facets of Cu,respectively,whose intensities enhanced with the increasing Cu content.
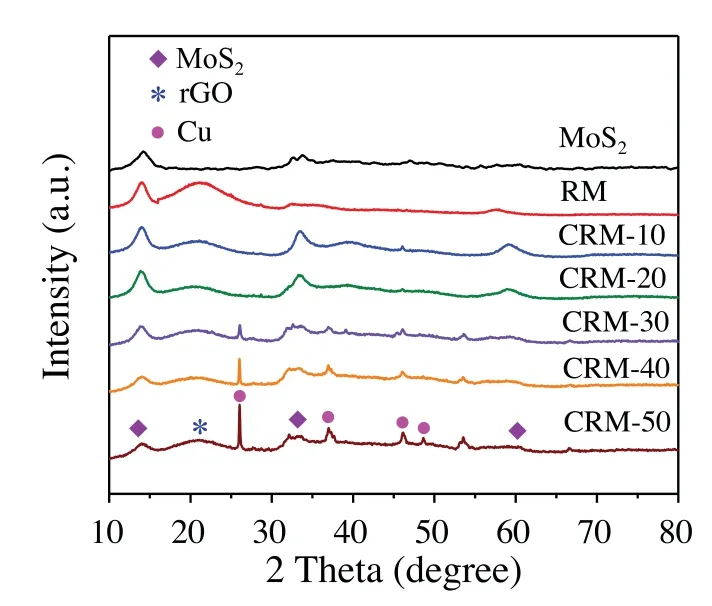
Fig.1.XRD spectra of MoS2, RM and CRM catalyst.
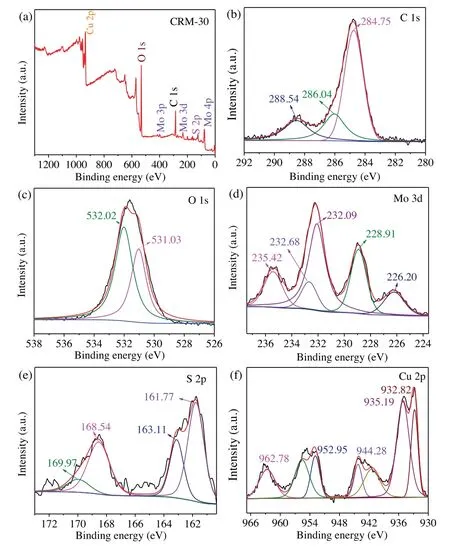
Fig.2.XPS of(a)survey spectrum,(b)C 1s,(c)O 1s,(d)Mo 3d,(e)S 2p and(f)Cu 2p of CRM-30 catalyst.
XPS was executed to further ascertain the details of the elemental composition and chemical bonding state of CRM-30.The full XPS survey spectrum (Fig.2a) revealed that the characteristic peaks of Mo, S, C, O and Cu as the main elemental components presented in CRM hybrid.The C 1s peak was deconvoluted into three peaks at 284.75 eV, 286.04 eV and 288.54 eV (Fig.2b) [34-36], which regarded to the C--C, C--O and O=C--O functional groups in the rGO,respectively[37].Fig.2c shows peaks at 531.03 and 532.02 eV corresponding to O 1s which can be attributed to the oxygen ingredient of the rGO.In Fig.2d, the binding energies of 228.91 and 232.68 eV corresponding to Mo 3d5/2and Mo 3d3/2indicated that Mo was in the form of Mo4+in MoS2.The peaks located at 235.42 and 232.09 eV were attributed to Mo 3d5/2and Mo 3d3/2of MoO3or MoO42-, which indicating the presence of Mo6+in the form of MoO3or MoO42-.For S 2p XPS spectrum(Fig.2e),two peaks centered at 161.77 and 163.11 eV were indexed to S 2p3/2and S 2p1/2of MoS2,respectively,indicating the presence of MoS2[38].And the peaks at 168.54 and 169.97 eV were the shake-up satellite.It was difficult to distinguish the Cu+and Cu0because of the close proximity of their energies.In Fig.2f,the peaks at 932.82 and 952.95 eV matched with Cu 2p3/2and Cu 2p1/2of Cu0,respectively.And the peaks of 942 and 962 eV corresponded to the satellite peaks.The binding energies at 935.19 and 944.28 eV corresponded to Cu 2p3/2and Cu 2p1/2of Cu+,respectively.It was probably that the excess NaBH4reduced Cu2+to Cu0, and the Cu0was partially oxidized to Cu+and Cu2+in the air[39].In summary,the synthesized MoS2nanosheets were well integrated with rGO and Cu in the mixture.The Cu content of CRM-30 was 30.39 wt%by inductive coupled plasma emission spectrometer (ICP) method(Table S2 in Supporting information).
Before photocatalysis, the optical band gap, adsorption and photoluminescence spectra are shown in Figs.S4,S5 and Table S3(Supporting information).As shown in Figs.3a and b,in 20 min of visible-light photocatalysis process using MoS2, the removal efficiency of RhB can reach over 90%.For the visible-light photocatalysis process using RM, RhB was removed 100% in 10 min.The modification of Cu showed obviously advantage for the photocatalytic reduction of RhB.Among CRM-10, 20, 30, 40, 50,GCM-30 displayed the best photocatalytic performance.The possible reason was high separation efficiency of photoelectronhole pairs.What's more,the amount of Cu affected the efficiency of charge transfer.Once it exceeded a certain amount,specific surface area of CRM will reduce, following an imperfect photocatalytic performance.As a representative sample, CRM-30 possesses higher photocurrent response relative than other photocatalysts,which clearly shows an efficient separation of photo-induced charge carriers[40].The pseudo-first-order kinetic model primely described the photocatalytic degradation kinetic process of RhB.It was found that the pseudo-first order reaction kinetics( k)of CRM-30 was reckoned 0.72 min-1, which was 3.79 times and 6.55 times higher than that of the RM (0.19 min-1) and MoS2(0.11 min-1),respectively.Fig.3c depict that the kinetic rate constant of CRM-30 was the highest among all samples.Fig.3d demonstrated the photocurrent-time curves of different photocatalysts.Taking RhB as a target, the photocatalytic activity of CRM was evaluated.In conclusion, the photocatalytic of ternary CRM-30 showed the superior catalytic activity and better stability(Fig.S6 in Supporting information).To identify the reactive oxygen species, p-benzoquinone (BQ), isopropanol (IPA) and EDTA-2 Na were selected as scavenger for superoxide radical anions (·O2-), hydroxyl radicals(·OH), hole (h+), respectively (Fig.S7 in Supporting information).
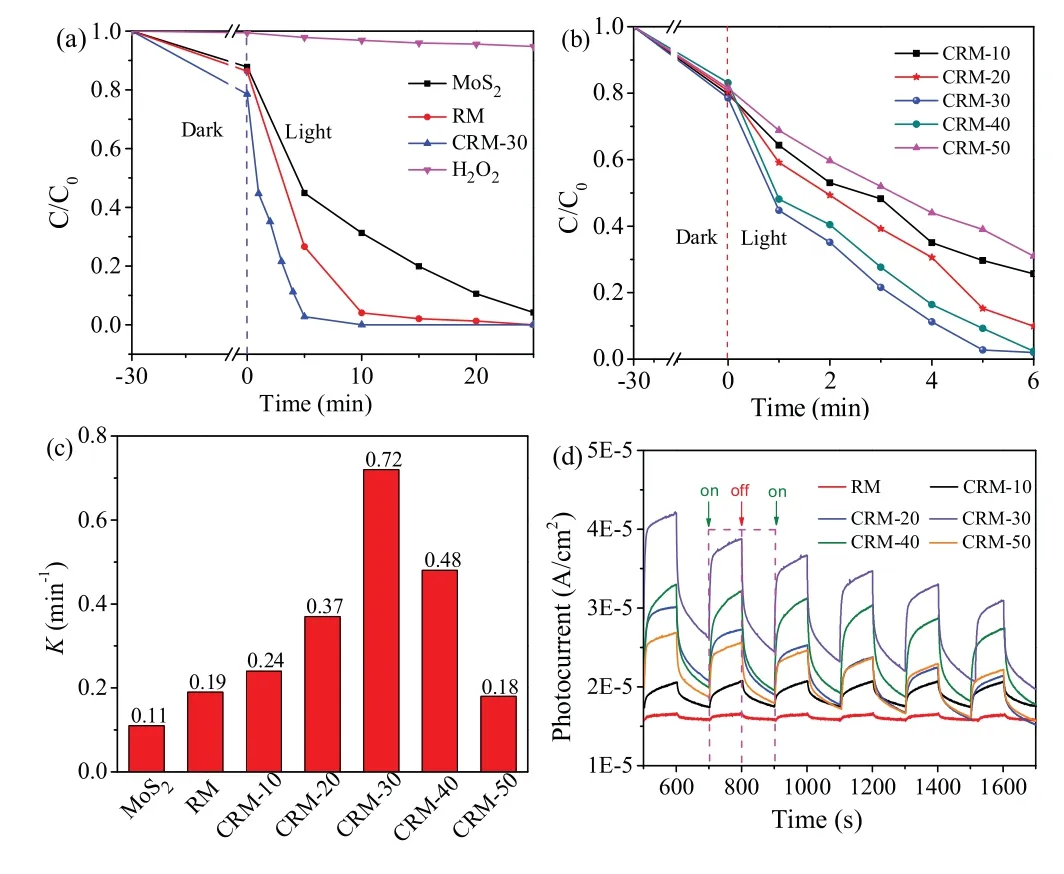
Fig.3.(a) The photocatalytic performance of MoS2, RM and CRM-30 catalyst.(b)The photocatalytic performance of CRM.(c)The pseudo-first-order degradation rate constants (k).(d) Transient photocurrent responses of different photocatalysts.
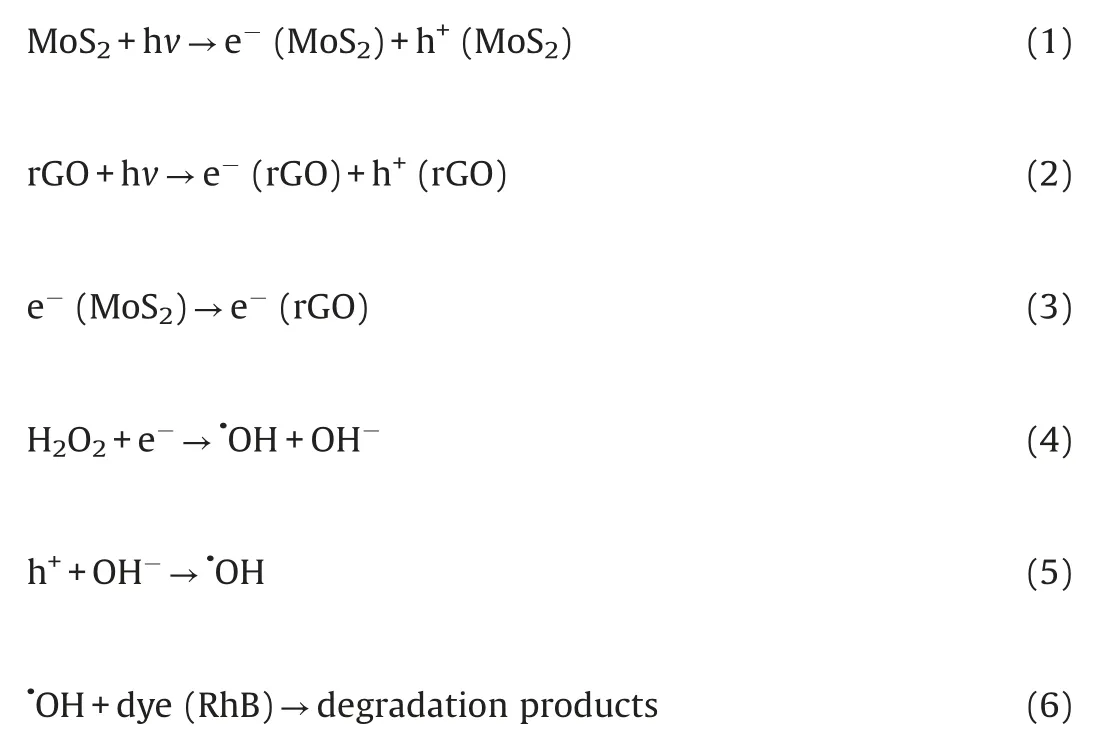
The photocatalytic reaction of CRM was illustrated in Scheme 2.The formation process of active radicals was expressed by the following Eqs.(1-6).As mentioned,the band gap of MoS2and CRM were 1.92 and 0.62 eV, respectively.Therefore, MoS2is easily excited by visible light illumination to generate electrons and holes.It is notable that the photogenerated electrons are rapidly transported from the conduction band(CB)of MoS2to rGO which displayed a momentous role in storing,promoting the separation,transfer and migration of charge carriers.Hence,the photocatalytic activity of RM was better than MoS2, and in the presence of the H2O2, the degradation rate is obviously accelerated.Moreover, Cu can improve the conductivity and may be worked as electron trap states and contribute to the consumption of electron [41,42].In other words, this synergy effect may accelerate the separation of electrons and holes.
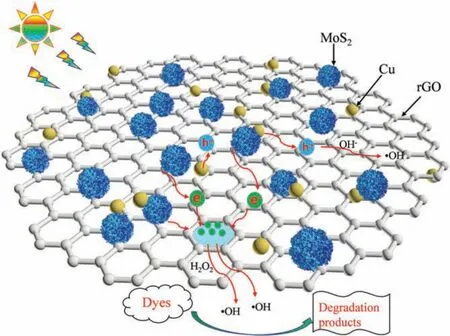
Scheme 2.Schematic drawing of the photocatalytic removal mechanism.
Cu/rGO/MoS2nanocomposites were successfully fabricated via a solvothermal and chemical method.MoS2was responsible for optical adsorption,rGO acted as a scaffold to anchor MoS2,and the load of Cu further contributed to separate the photogenerated charge carriers.Benefiting from the joint functions, Cu/rGO/MoS2exhibited superior photocatalytic performance and good stability towards degradation of organic dyes.This work will open a window for designing effective photocatalyst and promote the potential application of photocatalysis.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No.51878169), the Guangdong Innovation Team Project for Colleges and Universities (No.2016KCXTD023), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version, at https://doi.org/10.1016/j.cclet.2019.05.046.
 Chinese Chemical Letters2019年12期
Chinese Chemical Letters2019年12期
- Chinese Chemical Letters的其它文章
- Post-self-repair process of neuron cells under the influence of neutral and cationic nanoparticles
- CdS nanocrystallites sensitized ZnO nanorods with plasmon enhanced photoelectrochemical performance
- A simple visual method for DNA detection based on the formation of gold nanoparticles
- Self-assembly of L-tryptophan on Cu(111)studied by low-temperature scanning tunneling microscopy
- Functional delivery vehicle of organic nanoparticles in inorganic crystals
- Facile assembly of mesoporous silica nanoparticles with hierarchical pore structure for CO2 capture
