Design, synthesis and SAR study of novel sulfonylurea derivatives containing arylpyrimidine moieties as potential anti-phytopathogenic fungal agents
Wei Chen,Yuxin Li,Yunyun Zhou,Yi Ma,Zhengming Li
a School of Life Science and Engineering, Southwest Jiaotong University, Chengdu 610031, China
b State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China
c State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, Fudan University, Shanghai 200433, China
Keywords:
Sulfonylurea
Phytopathogenic fungi
Antifungal activity
Substituted pyrimidine
3D-QASR
ABSTRACT
Acetohydroxyacid synthase(AHAS)was considered as a promising target for antifungal agents.Herein,three series of novel sulfonylureas (SUs) 9-11 containing aromatic-substituted pyrimidines were designed and synthesized according to pharmacophore-combination and bioisosterism strategy.The in vitro fungicidal activities against ten phytopathogenic fungi indicated that most of the title compounds exhibited broad-spectrum and excellent fungicidal activities.Based on the preliminary fungicidal activities,a CoMFA model was constructed and the 3D-QSAR analysis indicated that either a bulky group around the 5-position of the pyrimidine ring or electropositive group around the 2-position of the benzene ring would be favour to fungicidal activities.In order to study interaction mechanism,10k was automatically docked into yeast AHAS and it further indicated that bearing bulky groups-aryl at the pyrimidine ring was critical to enhance antifungal activities.It revealed that the antifungal activity of derivatives 9-11 probably results from the inhibition of fungal AHAS.Thus,the present results strongly showed that SUs should be considered as lead compounds or model molecules to develop novel antiphytopathogenic fungal agents.
Plant diseases which mainly caused by phytopathogens have been recognized as a devastating threat to crop production,often lead to significant yield and quality reduction and economic losses in agriculture [1,2].Meanwhile, some of the fungi can produce mycotoxins harmful to animal and human health [3,4].Unfortunately, the control of plant pathogens is still a hard problem and the commercial chemical fungicides were not fully effective[5,6].Furthermore,with continuing use of these agrochemicals,residual toxicity, severe pesticide resistance and environmental pollution was emerged and evolved as a significant problem[7-9].Hence,it is necessary to develop new antifungal agents to control agricultural diseases [6,10,11].
Acetohydroxyacid synthase(AHAS,EC 2.2.1.6)catalyses the first common step in the branched chain amino acid(BCAA)biosynthesis pathway in fungi,bacteria and plants,but not in animals[12].The inhibition of AHAS interrupts the catalytic cycle and prevents the synthesis of BCAAs,which are essential for the subsistence of plants and microorganisms.Thus,AHAS has been regarded as an attractive target for fungicide, bactericide and herbicide discovery [13-16].Indeed, sulfonylureas (SUs) have been confirmed as ultra-highly efficient and high selectivity herbicide targeting AHAS since the late 1970s[17].Subsequently,Pang et al.reported the crystal structures of Saccharomyces cerevisiae AHAS and Arabidopsis thaliana AHAS as the free enzyme and in complex with SUs [18-21].These studies have elucidated the molecular basis for the inhibition of SUs toward AHAS,andprovideduswithpowerfulinformationtorationaldesign novel antifungal agents.Lee et al.reported that when the gene for AHAS was mutated in Cryptococcus neoformans and Candida albicans, these fungi became attenuation of virulence due to the lack of BCAAs,further showing that AHAS is a promising target for antifungal agents [16,22,23].In addition, some compounds have been identified for antituberculosis activity targeting AHAS,suggesting that AHAS is a potential target for the research of antimicrobial chemotherapy[24,25].Nevertheless,research on SUs for agricultural fungicide has been limited for decades.
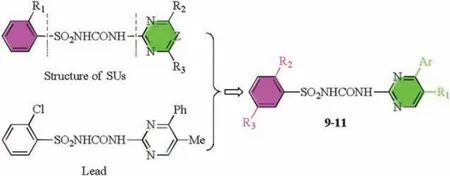
Fig.1.Design strategy of compounds 9-11.
In general,SUs compose by one ortho-substituted benzene ring,sulfonylurea bridge and one heterocyclic ring(either a pyrimidine(Z=CH)or triazine(Z=N))(Fig.1).As literatures showed that SUs'activities were vigorously impacted by heterocycles or substitutents[12,26].Additionally,pyrimidine was a key moiety in a wide variety of bioactive molecules including antifungal,antimicrobial,anticancer and anti-inflammatory agents [27,28].In our precious work, Chen et al.found that some SUs containing a bulk group at pyrimidine moiety exhibited moderate to good inhibitory rates against plant pathogenic fungi [29,30].Wei et al.also reported some sulfonylureas containing an alkenyl moiety at benzene ring exhibited enhanced antifungal activities than chlorsulfuron [31].
Herein, in an effort to discover increasingly potent antifungal agents,great importance had been focused on the modifications to the pyrimidine group.This work present rational designed and synthesized novel SU derivatives 9-11 based on the lead compounds (Fig.1), and their antifungal activity was evaluated in vitro.The structure-activity relationships were studied through comparative field analysis(CoMFA)model,from which we tried to identify the key structural factors influence on inhibitory activity.In addition,molecular docking was carried out to study the binding modes of 10k with yeast AHAS and explain the potential antifungal mechanism of the title compounds.
As shown in Schemes S1 and S2(Supporting information),N,Ndimethylformamide dimethyl acetal was refluxed with aryl ethanones (1a-1c) for 4 h and then condensed with guanidine hydrochloride to generated the crude products 3a-3c,followed by recrystallize from ethanol/hexane and obtained the arylpyrimidines [29].4a-4c and 5a-5c were facilely prepared from 3 with corresponding N-halosuccinimide under reflux acetonitrile in reasonable yield, and 6a-6c were obtained under a lower temperature (40°C) [32].The key intermediates 4-6 were confirmed by 1D and 2D NMR spectra (Figs.S1-S3 in Supporting information)and indicated that halogenation of pyrimidines 3 was regioselective occurred at the H-3 position and obtained the corresponding products (Fig.S4 in Supporting information).The sulfonyl carbamates 8a-8d were prepared in excellent yield by treating sulfonamides 7a-7d with ethyl chloroformate in the presence of powdered potassium carbonate in refluxing acetone.With intermediates 3-6 and 8 in hand,the title compounds 9-11 were synthesized and purity according to the literatures in 60%-80% yield [29,33].
The in vitro inhibitory activity of compounds 9-11 was tested against ten phytopathogenic fungi at the dosage of 50 mg/L.The screening data(Table S1 in Supporting information)indicated that most of the tested compounds showed broad-spectrum and excellent fungicidal activities.For every fungus,all or some of the compounds were found to be more active than model compounds or positive controls.In particular, 9j, 9m, 9n, 10k, 11d and 11e showed 87.5%,87.5%,87.5%,87.5%,80.0%and 87.5%inhibitory rate against C.arachidicola,respectively,which were much higher than chlorothalonil (75.0%), carbendazim (<50%) and lead compound(≤50%).For C.gramineum, 9e, 9i, 9k, 9 l, 9n, 10f, 11i and 11k exhibited >90% antifungal activity and comparable to chlorothalonil (100%) and carbendazim (100%).9i,11e,11i and 11 j showed more than 80%inhibitory rate against 6 fungi or even more.Over 14 title compounds showed as high as 80% inhibition rate against C.gramineum and R.solani.Furthermore, the EC50values of 11 SUs against C.gramineum were equal to or better than chlorothalonil(0.016μmol/L), 10k exhibited the best value (0.009μmol/L,highlighted in Table S2 in Supporting information).
The preliminary results in Table S1 showed that introduce bulk groups-aryl (p-toyl, 2-furyl and 2-thienyl) to pyrimidinyl 4-position in an obvious improve the antifungal activities and broaden the antifungal spectrum compared to sulfometuronmethyl(≤50%).Comparing the fungicidal activities of 9-11 against all the fungi, it exhibited that derivatives 11 had a little more activities and broader spectra than 9 and 10.It further demonstrated that substituents at 4-position of pyrimidine ring will influence the antifungal activities.In addition,the existence of a halogen atom at pyrimidinyl 5-position was superior to a hydrogen(9e-9p versus 9a-9d),the approximately order is R1=Br>R1=Cl >R1=I >R1=H.
In comparison, the substituents at the benzene ring have a direct relationship between antifungal activities.Such as in series 11, the 2-NO2compounds exhibited higher activities and more broaden spectra than the analogues (11e versus 11f,11 g,11h; 11i versus 11k,11 l and 11 m versus 11n,11o,11p),with the exception of 11j which was as effective as 11i.It also indicated that introducing nitro to the 5-position of benzene ring will decrease the antifungal activities.Such as 9f (R2=Cl, R3=NO2) displayed much lower antifungal activity and narrower spectra than 9e (R2=Cl).In summary, the variation of substituents on the pyrimidine moiety has much more effect on the fungicidal activity.These results provide useful information for further optimization of the compounds.
CoMFA analysis was performed on the EC50of compounds 9-11 against C.gramineum and started from the structural alignment(Fig.S5 in Supporting information).According to Table S2,10k had the lowest EC50value and therefore was selected as the template molecule.9d,9 g,9p,10o and 11 g were picked randomly as the test for model validation.The best CoMFA model was established with q2=0.662(cross-validated coefficient)and r2=0.879(conventional correlation coefficient).The contributions of steric and electrostatic fields are 85.3% and 14.7%, respectively.And the steric field is played as the main contribution in this model.The calculated activity values are fairly accurately with the observed,indicating that this final model exhibits excellent predictability on these compounds (Table S2).
The steric contour was plotted in Fig.S6a (Supporting information).A large green contour surrounding the 4-methylphenyl group,indicating that the introduction of a bulky group at this site is conducive to increase antifungal activity.Such as compounds 9e-9p (R1=Cl, Br, I) has a higher inhibitory activity than compounds 9a-9d(R1=H).The electrostatic fields of CoMFA were shown in Fig.S6b(Supporting information),the blue contour defines a region where a decrease in the electron density is favorable, whereas the red contour means the contrary.A characteristic feature is the presence of a big and medium-sized blue contour around the 2-position of benzene ring and two bigsized surrounding the 5-position of pyrimidine ring, which is favour to activity.Meanwhile, there is also a smaller red contour near the substituents of pyrimidine ring,so the introduced groups should not be too electron-deficient.It is reflected in certain compounds, such as 9i (R1=Br, R2=NO2),10k (R1=Br,R2=Cl) and 11e (R1=Cl, R2=NO2) exhibited excellent activity.
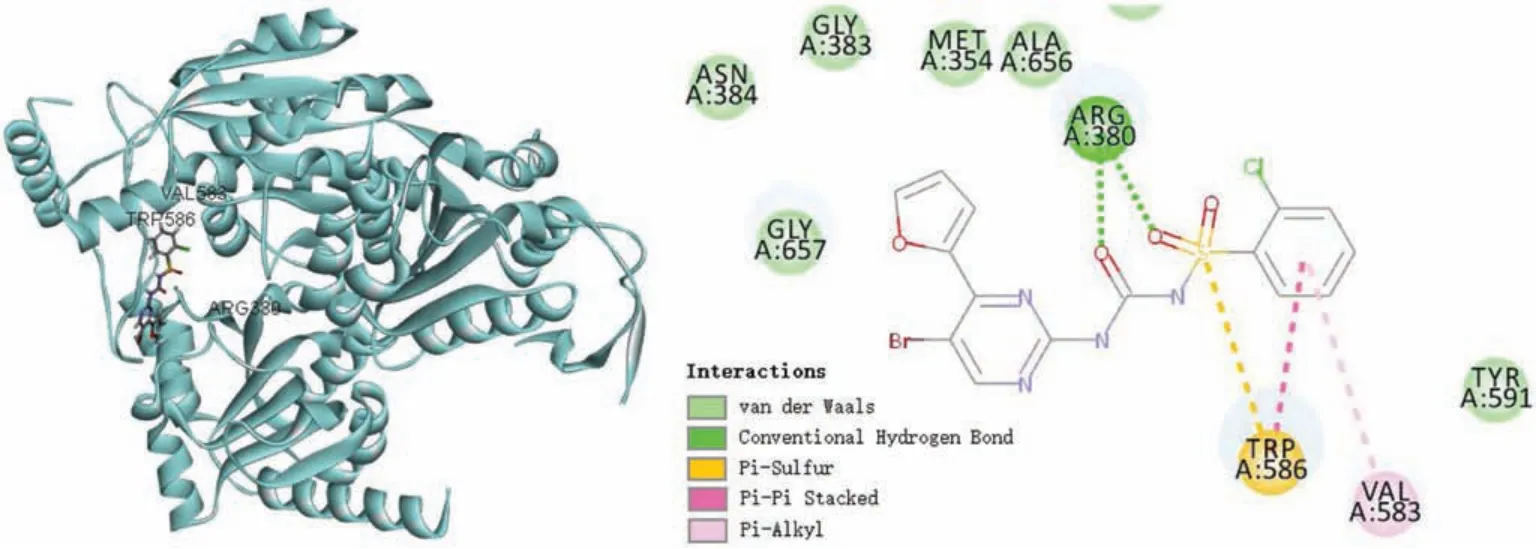
Fig.2.Docking mode of 10k to yeast AHAS.
The flexible molecular docking model was generated via 10k and yeast AHAS(PDB code:1T9B).As shown in Fig.2,the binding model is remarkable resemble yeast AHAS in complex with chlorsulfuron.10k is inserted into the entrance of hydrophobic tunnel with the 4-(2-furyl)-pyrimidine ring projecting toward the active site, while the aromatic ring is bound in a pocket near the surface of the protein.Docking results showed that the negative Cdocker_Interaction_Energy was 32.04 kcal/mol and the negative Cdocker_Energy was 13.85 kcal/mol.It mainly formed two hydrogen bonds between the oxygen atom of the sulfonyl group with the amino group of Arg 380.The preference for bulky groups on the pyrimidine could be explain by the van der Waals interactions exist between 10k with AHAS (Gly 657, Asn 384, Gly 383, Met 354,Ala 656, etc.).It suggested that aryl substituents can perfectly embed within the active pocket and profitable for antifungal activties.The π-π stacking interactions between 10k and the residues of Trp 586 and Val 583 also play important roles for their high inhibitory activities.The results of molecular docking indicated the potential antifungal mechanism of the title compounds 9-11.
In summary, 48 novel sulfonylureas 9-11 containing arylpyrimidine moieties were designed, synthesized and evaluated against phytopathogenic fungal.Their structures were characterized by1H NMR,13C NMR, HMBC and HRMS.The fungicidal activities showed that most the title compounds exhibited broadspectrum and excellent fungicidal activities.Furthermore,the EC50value of 10k(0.009 μmol/L)against C.gramineum was much better than chlorothalonil (0.016μmol/L).Based on the preliminary fungicidal activities, a CoMFA calculation was carried out and the 3D-QSAR analysis indicated that either a bulky group around the 5-position of the pyrimidine ring or electropositive group around the 4-position of the pyrimidine ring and the 2-position of the benzene ring would have the potential to improve the fungicidal activities.In order to further study the antifungal interaction of derivatives 9-11.10k was automatically docked into yeast AHAS and the Cdocker is mainly stabilized by several intermolecular interactions including hydrogen bonds, π-π stacking and van der Waals interactions.It further indicated that bearing bulky groups-aryl at the pyrimidine ring was critical to enhance antifungal activities and inferred that antifungal activity of compounds 9-11 probably results from the inhibition of fungal AHAS.The theoretical and experimental results provide reference for developing SUs as new fungicides.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21702173, 21502229), Fundamental Research Funds for the Central Universities (No.2682016CX104)and Miaozi Project of Scientific and Technological Innovation of Sichuan Province (No.2018090).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.04.072.
 Chinese Chemical Letters2019年12期
Chinese Chemical Letters2019年12期
- Chinese Chemical Letters的其它文章
- Post-self-repair process of neuron cells under the influence of neutral and cationic nanoparticles
- CdS nanocrystallites sensitized ZnO nanorods with plasmon enhanced photoelectrochemical performance
- A simple visual method for DNA detection based on the formation of gold nanoparticles
- Self-assembly of L-tryptophan on Cu(111)studied by low-temperature scanning tunneling microscopy
- Functional delivery vehicle of organic nanoparticles in inorganic crystals
- Facile assembly of mesoporous silica nanoparticles with hierarchical pore structure for CO2 capture
