Mesoporous ZSM-5 Crystals Synthesized by a Novel Amphiphilic Organosilane Soft Template
WANGYumin(王毓敏),LIUDongliang(劉棟良)
Department of Applied Chemistry, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, China
Abstract: Hierarchical ZSM-5 zeolite was hydrothermally synthesized by a novel silicone quaternary ammonium salt surfactant as a mesopore director. The crystallinity and the morphology of sample were characterized by X-ray diffraction (XRD), field-emission scanning electron microscope (FE-SEM), and transmission electronic microscope (TEM). Results indicated that the prepared ZSM-5 zeolites had good crystallinity, ellipsoid structure morphology and rough surface. Moreover, the pore size was enlarged to about 4 nm measured by nitrogen sorption, which indicated positive function of the synthesized template.
Key words: hierarchical pore; ZSM-5 zeolite; soft template; organosilane
Introduction
Zeolites are a family of crystalline aluminosilicate materials whose diameters are less than 2 nm[1]. They have significant applications in the fields of catalysis, organic synthesis and petrochemical industry[2-6]owing to their high specific surface area, strong adsorption capacity, high thermal and hydrothermal stability[7-8]. However, their small pore size causes diffusion limitations for bulky molecules, which limits their application[9-11].
Up to now, many researches have been carried out to solve this problem. One of the typical approaches is to direct the synthesis of mesoporous zeolites by templates. The key of this method is that these templates have strong interaction with inorganic precursors, which can effectively modulate the crystallization process of inorganic materials[12-19]. Choetal. succeeded in preparing beta zeolites through using a dual structure-directing organosilanes surfactant as a mesopore-directing agent[20]. Xuetal. designed a single quaternary ammonium head amphiphilic template which contained aromatic groups to direct forming meso-structured zeolites[21].
Inspired by soft templating agent-oriented synthesis of mesoporous zeolites, we herein designed and synthesized a new amphiphilic organosilane template N, N-dimethoxycarbonylethyl-N-decyl-3-trimethoxysilyl) propan-1-aminium iodide (DDTPAI) to direct the synthesis of mesoporous ZSM-5 zeolite, whose chemical structure was shown in Fig. 1. This template possesses a surfactant-like long chain alkylammonium, a hydrolysable methoxysilyl group and a dimethoxycarbonylethyl section. The part of hydrophobic alkyl chain and dimethoxycarbonylethyl section occupy a larger space, which theoretically can create larger channels by interacting with the inorganic precursors. The hydrolysable methoxysilyl group is ready to react with the silicon source, which is beneficial to forming crystalline mesopores.
Hierarchical ZSM-5 zeolite was prepared by a one-step method, templated by the synthesized template DDTPAI. The results showed that DDTPAI expressed the ability to direct synthesis of hierarchical ZSM-5 zeolites. The prepared zeolite contained intracrystalline mesoporosity and its pore size was about 4 nm.
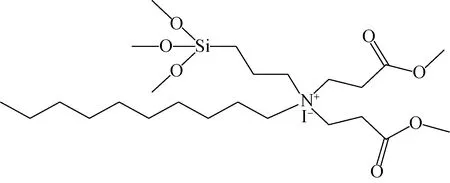
Fig. 1 Molecular structure of the synthesized organosilane template DDTPAI
1 Experiments
The synthesis of DDTPAI is divided into two steps: first, tertiary amine is prepared by Michael addition; then, the desired ammonium salt is synthesized under nitrogen protection.
1.1 Materials
1-Decylamine was an analytical reagent and purchased from Tokyo Chemical Industry, Japan. Dry ethanol was an alytical reagent and purchased from Hongsheng Fine Chemical Co., Ltd., China. Ethyl acetate and hexane were analytical reagents and purchased from Tansoole Technology Co., Ltd., China. (3-Iodopropyl) trimethoxysilane was of 95% purity and purchased from J&K Chemical Co., Ltd., China. Methyl acrylate, acetone, petroleum ether (60-90 ℃), N, N-dimethylformamide(DMF), aluminum isopropoxide, and tetraethyl orthosilicate were analytical reagent, and purchased from the Sinopharm Chemical Reagent Co., Ltd., China. Tetrapropylammonium hydroxide (TPAOH, 25% by weight aqueous solution) was purchased from the Sinopharm Chemical Reagent Co., Ltd., China.
1.2 Preparation of tertiary amine
For the synthesis of tertiary amine with C10length, 1-decylamine (0.05 mol, 7.87 g) was slowly added into a solution of methyl acrylate (0.15 mol, 12.91 g) dissolved in dry ethanol (50 mL). The reaction mixture was stirred at room temperature for 5 h. Then, ethanol was evaporated and the crude product was purified by column chromatography using ethyl acetate, and the obtained light yellow liquid was the target product N, N-dimethoxycarbonylethyl-decylamine (yield: 67%).
1.3 Characterization of tertiary amine
The structure of tertiary amine was confirmed by thin layer chromatography (TLC), ESI-MS,1H NMR and13C NMR spectra. The data are as follows.
Rf=0.75 (acetone/petroleum ether = 1∶2 in vol.).
ESI-MS m/z for C18H35NO4([M+H]+) found: 330.2, calculated: 330.3.
1H NMR (400 MHz, CDCl3): δ [0.88 (t,J=8 Hz, 3 H, (CH2)8CH3)], [1.24-1.44 (m, 16 H, (CH2)8CH3)], [2.40 (t,J=8 Hz, 2 H, CH2(CH2)8)], [2.44 (t,J=8 Hz, 4 H, 2×CH2CO)], [2.76 (t,J=8 Hz, 4 H, 2×NCH2)], [3.66 (s, 6 H, 2×OCH3)].
13C NMR (100 MHz, CDCl3): δ 13.9 (CH3), 22.2-31.8 ((CH2)8), 32.4 (CH2CO), 49.2 (N(CH2)2), 51.2 (OCH3), 53.7 (NCH2(CH2)8), 172.8 (CO).
1.4 Synthesis of DDTPAI
To synthesize DDTPAI, N, N-dimethoxycarbonylethyl-decylamine (0.005 mol, 1.65 g) and (3-iodopropyl)trimethoxysilane (0.005 5 mol, 1.60 g) were dissolved in the DMF (4 mL) , stirred at the temperature of 120 ℃ for 12 h under nitrogen and then cooled to the room temperature. Then, the solvent was evaporated, and the rough product was washed with hexane and dried under high vacuum oven at 50 ℃ for 2 h.
1.5 Characterization of DDTPAI
The structure of DDTPAI was confirmed by TLC and13C NMR spectra.
Rf=0 (acetone/petroleum ether = 1∶2 in vol.).
13C NMR (100 MHz): δ 33.1-33.6 (CH2), 58.0 (OCH3), 60.5-66.8 (NCH2), 179.2 (CO).
1.6 Synthesis of mesoporous zeolite
A mixed solution involving 0.040 8 g aluminum isopropoxide (AIP), 2.60 g tetrapropylammonium hydroxide (TPAOH, 25 wt% aqueous solution) and 7.20 g deionized water was first stirred in a 250 mL three-necked flask for about 30 min, after which 2.08 g tetraethyl orthosilicate (TEOS) was added and the mixture was stirred at room temperature for 3 h. Then, 0.31 g DDTPAI was added, after which the mixture was further vigorously stirred for another 4 h. The resultant aluminosilicate gel had a molar composition of 1 SiO2/0.01 Al2O3/0.32 TPAOH/40 H2O/0.05 DDTPAI. The aluminosilicate gel was transferred into an autoclave lined with polytetrafluoroethylene and heated in an oven at 150 ℃ for 3 d. The products obtained were collected by filtration with deionized water, dried in an oven at 150 ℃ for 3 h and calcined at 600 ℃ for 5 h with a heating rate of 1 K/min to remove the template. The so-obtained product was denoted as ZSM-5-D. Similarly, the conventional ZSM-5 was synthesized by the same method except for the addition of DDTPAI.
2 Results and Discussion
2.1 X-ray diffraction (XRD) analysis
The powder XRD pattern of the mesoporous zeolite ZSM-5-D is shown in Fig. 2, which confirms the intact crystalline structure and manifests the typical peaks of MFI structure in the region of 2θ(5°-40°). Meanwhile, it is worth noting that the absence of a halo indicates no other crystalline phases are detected. All of these demonstrate the positive contribution of template DDTPAI to the synthesis of mesoporous zeolites is reasonably concluded.
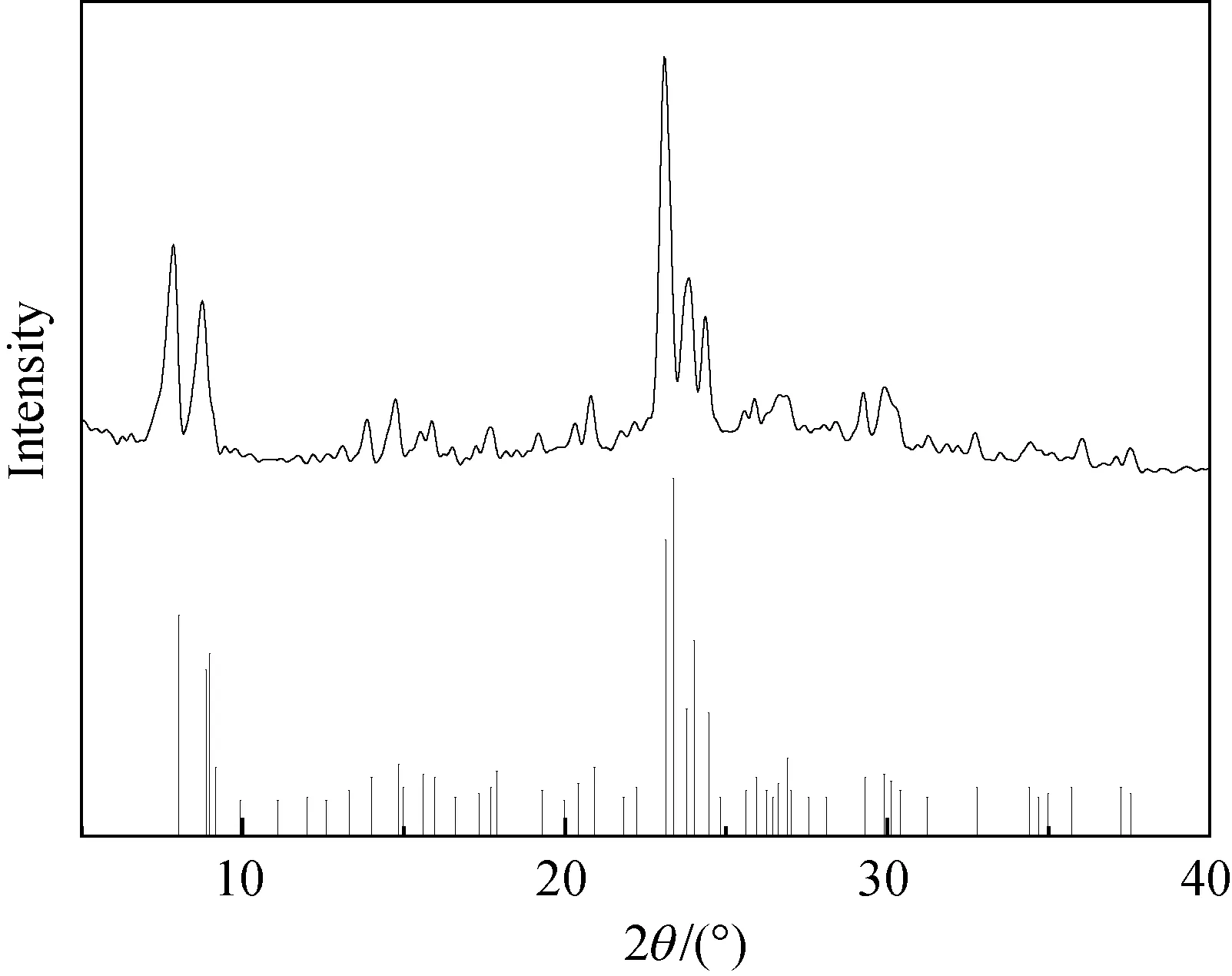
Fig. 2 XRD patterns of ZSM-5-D (for structural comparison, a “standard” XRD pattern of ZSM-5 (ICSD 201183) was listed at the bottom)
2.2 Field-emission scanning electron microscope (FE-SEM) and transmission electronic microscope (TEM) analysis
Figure 3 shows the FE-SEM images of ZSM-5-D and ZSM-5. The ellipsoid structures are observed in the sample ZSM-5-D [Figs. 3(a) and 3(b)], while conventional zeolite ZSM-5 exhibits a pie-like stacked morphology. Moreover, the rough surface of ZSM-5-D could be clearly observed as shown in Fig. 3(c), compared with the smooth surface of conventional ZSM-5. The TEM image recorded from ZSM-5-D is shown in Fig. 4(a), which contains some holes, indicating the presence of mesopores in the sample. Additionally, the apparent lattice fringes in Fig. 4(b) provide direct evidence for the good crystallinity in the hierarchical zeolite ZSM-5-D. All of these results reflect that the addition of the organosilane template leads to a significant change in the morphology of ZSM-5 crystal.
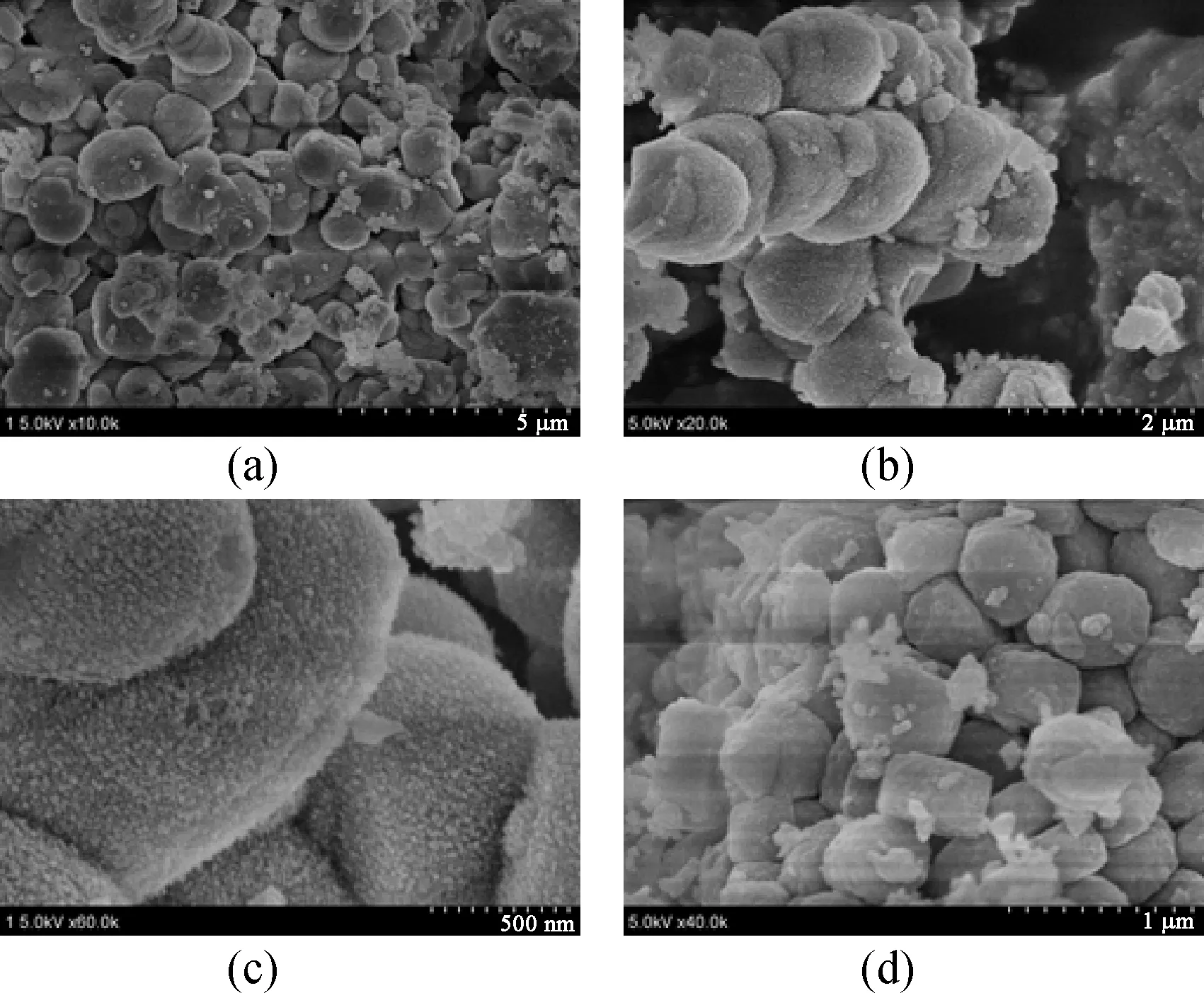
Fig. 3 SEM images of as-synthesized products: (a), (b) and (c) hierarchical zeolite ZSM-5-D at different magnifications; (d) conventional ZSM-5 zeolite synthesized in the absence of DDTPAI
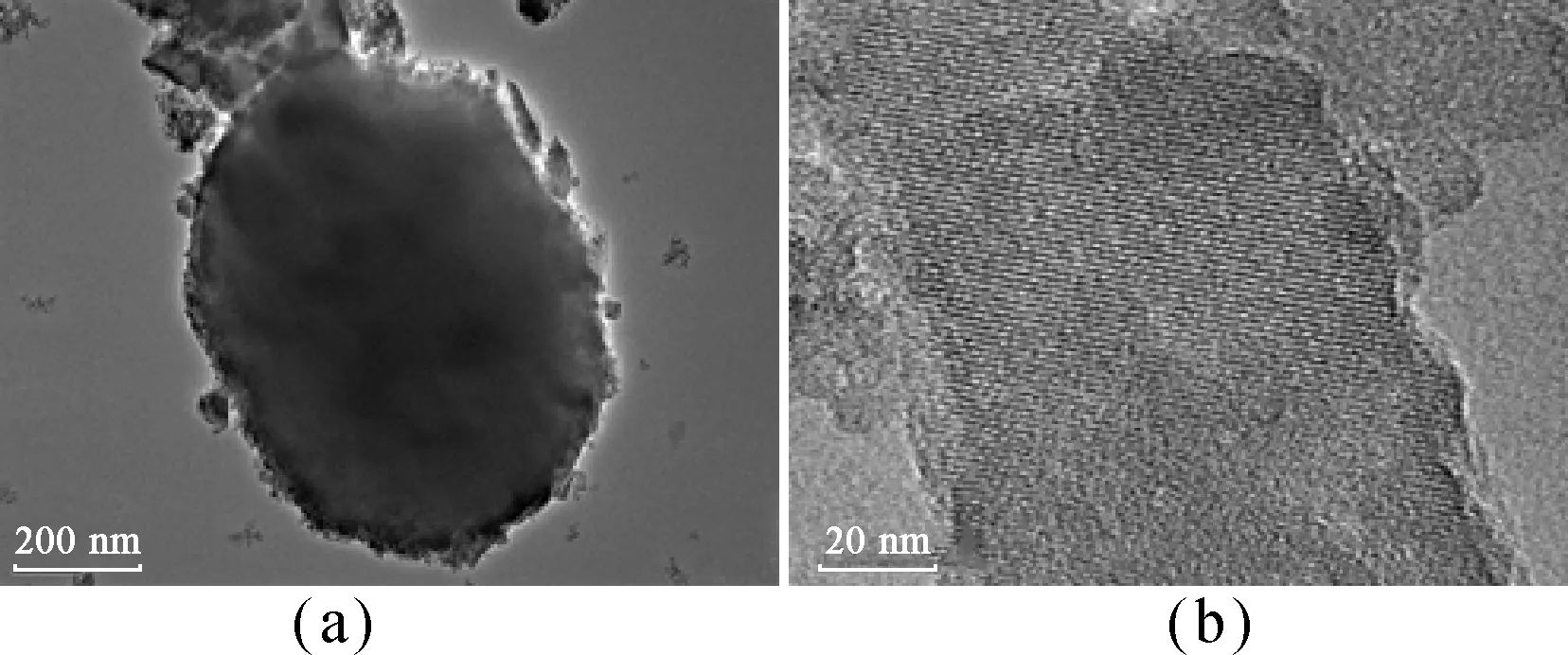
Fig. 4 TEM images of ZSM-5-D: (a) in low-magnification; (b) in high-magnification
2.3 Nitrogen sorption analysis
To get more detailed information about the pore structure, nitrogen adsorption-desorption isotherms and DFT pore size distribution of ZSM-5-D are obtained as shown in Fig. 5. It can be obviously observed in Fig. 5(a) that there is a typical type IV hysteresis loop in the range of 0.4
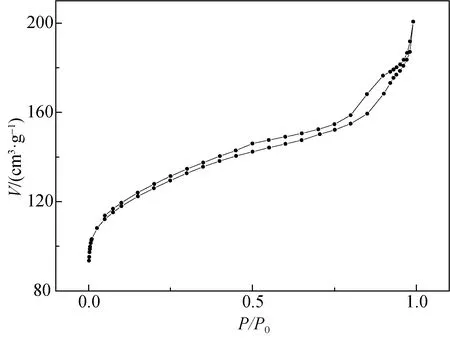
(a)

(b)
3 Conclusions
ZSM-5 zeolite, prepared by a novel organosilane template DDTPAI as a mesopore director, contains intracrystalline mesoporosity and its pore size is enlarged to about 4 nm. Structure and morphology analyses with XRD, SEM, TEM, and nitrogen sorption demonstrate the presence of mesopores. The unique pore system in ZSM-5-D may be useful for the diffusion and mass transport, which is important for catalytic performance involving bulky molecules.
 Journal of Donghua University(English Edition)2019年4期
Journal of Donghua University(English Edition)2019年4期
- Journal of Donghua University(English Edition)的其它文章
- Parameter Estimation for Complex Ornstein-Uhlenbeck Processes
- Elastic Predictions of 3D Orthogonal Woven Composites Using Micro/meso-scale Repeated Unit Cell Models
- Sound Character Calculation and Analysis of Sound Barrier Based on Acoustoelectric Analogy
- Tool Health Condition Recognition Method for High Speed Milling of Titanium Alloy Based on Principal Component Analysis (PCA) and Long Short Term Memory (LSTM)
- Upper Bound Solution of Soil Slope Stability under Coupling Effect of Rainfall and Earthquake
- Exploring the Performance of Magnetic Immobilized Lysozyme on Sludge Hydrolysis and Mechanism of Improving Dewaterability of Excess Sludge
