Crizotinib-induced acute fatal liver failure in an Asian ALK-positive lung adenocarcinoma patient with liver metastasis: A case report
Ying Zhang, Yan-Yan Xu, Yi Chen, Jin-Na Li, Ying Wang
Abstract
Key words: Fatal liver failure; Crizotinib hepatotoxicity; Liver metastases; ALK rearrangement; Lung adenocarcinoma; Case report
INTRODUCTION
Lung cancer is one of the most common cancers worldwide, and it is the leading cause of cancer deaths. Non-small-cell lung cancer (NSCLC) accounts for nearly 80%-85% of lung cancers[1-5]. Most NSCLCs are unresectable and are already advanced upon diagnosis[3]. Molecular target therapy is effective for advanced NSCLC patients with related gene mutations. Some specific driver mutations in genes, including epidermal growth factor receptor, Kirsten rat sarcoma viral oncogene and anaplastic lymphoma kinase (ALK), have been identified in NSCLC[6].ALKrearrangements are defined as a special molecular subtype of lung cancer and have been found in 5%-7% of NSCLC patients[7].
Crizotinib (XalkoriTM, Pfizer) is a multi-target tyrosine kinase inhibitor that targetsALK, mesenchymal epithelial transition and c-ros oncogene 1 receptor tyrosine kinase.It was approved as a first-line therapy for the treatment ofALK-rearranged NSCLC by the Food and Drug Administration in 2011[8]. Crizotinib showed an improved survival rate compared to conventional chemotherapy in patients with advanced or metastaticALK-positive NSCLC[9-11]. Common adverse effects of crizotinib have been reported,including vision disorders, gastrointestinal disturbances, electrocardiographic abnormalities, hypogonadism and hepatotoxicity. In five clinical trials (PROFILE 1001[12], 1005[13], 1007[10], 1014[14], 1029[15]), elevated aminotransferases were observed in 10%-38% of patients. Among them, 2%-16% of grade 3 or 4 patients had increased aminotransferase levels. However, most transaminase abnormality was reversible.Only 0.4% of patients exhibited irreversible hepatotoxicity, and two of them died[16].Herein, we report a case of acute fatal crizotinib-induced liver failure when crizotinib was used as a first-line therapy for hepatic metastases in an Asian patient with lung adenocarcinoma. In addition, a literature review on crizotinib-induced hepatitis is presented.
CASE PRESENTATION
Chief complaints
A 37-year-old Asian man complained of dyspnea and upper abdominal pain for a week in August 2017.
History of present illness
The patient was admitted to our hospital in July 2017 due to complaints of fever and severe cough. Positron emission tomography-computed tomography (PET-CT)identified a primary malignant nodule on the right lower lung lobe and mediastinal metastasis without liver metastasis. The patient underwent successful resection of the primary pulmonary nodules. Pathological examination showed a lung adenocarcinoma with echinoderm microtubule associated protein-like 4 andALKrearrangement measured by fluorescence in situ hybridization. The patient did not receive chemotherapy after the operation due to his faint physical condition. In August 2017, he presented with dyspnea and upper abdomen pain for a week and returned to the hospital.
History of past illness
There was no history of past illness.
Personal and family history
He was a non-smoker and non-drinker, also without history of drug allergy.
Physical examination upon admission
A physical examination revealed hepatomegaly and liver tenderness, and the Karnofsky performance status (KPS) was a score of 50.
Laboratory examinations
On examination (day 1), a full panel of liver function tests revealed that the prothrombin time (PT), serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were elevated, as follows: PT: 17.3 s (normal reference< 12.5 s), AST: 95 IU/L (normal reference < 34 IU/L), ALT: 55 IU/L (normal reference< 40 IU/L). However, the total bilirubin (10.6 μmol/L) level was normal (normal reference < 20.5 μmol/L). Biliary obstruction, non-alcoholic fatty liver disease,ischemic hepatitis and hepatitis A, B or C virus infection were excluded. Serum tests were negative for Cytomegalovirus, Epstein-Barr virus, Herpes Simplex, hepatitis E virus antibodies and anti-mitochondrial smooth muscle. Other laboratory data were within normal limits, including serum ferritin, copper and ceruloplasmin.
Imaging examinations
Chest and abdominal CT scans identified an 8.4 cm × 9.8 cm mediastinal metastatic lump and multiple hepatic metastases as a baseline (Figure 1A and D).
FINAL DIAGNOSIS
After consideration of the patient's present history of lung adenocarcinoma and CT scans, the final diagnosis was advancedALK-positive lung adenocarcinoma with multiple distant metastases (cT1N2M1b, Stage IV).
TREATMENT
The patient received 250 mg oral crizotinib twice daily as first-line therapy and 456 mg polyene phosphatidylcholine capsules three times daily for transaminase elevation. On day 10, serum AST (36 IU/L) and ALT (58 IU/L) levels were obviously decreased. Clinical signs of dyspnea were relieved and upper abdomen pain disappeared by day 18. KPS was reevaluated, and the score was 90. Chest and abdominal CT scans identified the stable mediastinal lump and multiple hepatic nodules. On day 25, liver enzyme levels had decreased to normal values. On day 35,chest and abdominal CT scans revealed a slight decrease in volume of mediastinal and liver metastases (Figure 1B, 1E). The therapeutic effect was classified as stable disease according to the Response Evaluation Criteria in Solid Tumors. On day 46, the patient was found to have icteric sclera and abnormal liver enzyme levels during his outpatient follow-up, but he refused treatment. On day 55, he began to appear drowsy but with normal vital signs. Physical examination showed icteric sclera and asterixis. Laboratory data indicated liver function impairment, as follows: AST: 402 IU/L, ALT: 215 IU/L, total bilirubin: 145 μmol/L; PT: 18 s. ALT level, AST level and total bilirubin were evaluated as toxicity grade 3. The plasma NH3 (63 μmol/L) level accumulated (9 < normal reference < 33 μmol/L). Chest CT scan showed that the mediastinal lump was unchanged (Figure 1C) and head CT scan was normal.However, abdominal CT scan revealed acute intrahepatic bile duct dilatation, massive ascites and an increase in liver volume by 20% with unvaried metastatic nodules(Figure 1F). According to the King's College Criteria, fulminant liver failure and hepatic encephalopathy were diagnosed. Crizotinib-induced liver failure was strongly suspected, and crizotinib treatment was discontinued from the evening of day 55. The patient was given intensive treatments for acute hepatic failure, including lactulose against encephalopathy, prophylactic antibiotics, proton pump inhibitor, human albumin and plasma, according to the practice guidelines. On day 57, his disturbed consciousness improved, and the level of AST (150 IU/L), ALT (129 IU/L) and total bilirubin (99.1 μmol/L) decreased. Although these intensive therapies were continued, his liver function rapidly deteriorated, as follows: AST: 1075 IU/L, ALT:240 IU/L, total bilirubin: 233 μmol/L; PT: 29 s, NH3: 163 μmol/L. The changes in AST, ALT and total bilirubin from day 1 to day 59 are shown in Figure 2. He went comatose on day 59.
OUTCOME AND FOLLOW-UP
On day 60, the patient died. Autopsy or liver biopsy could not be conducted.
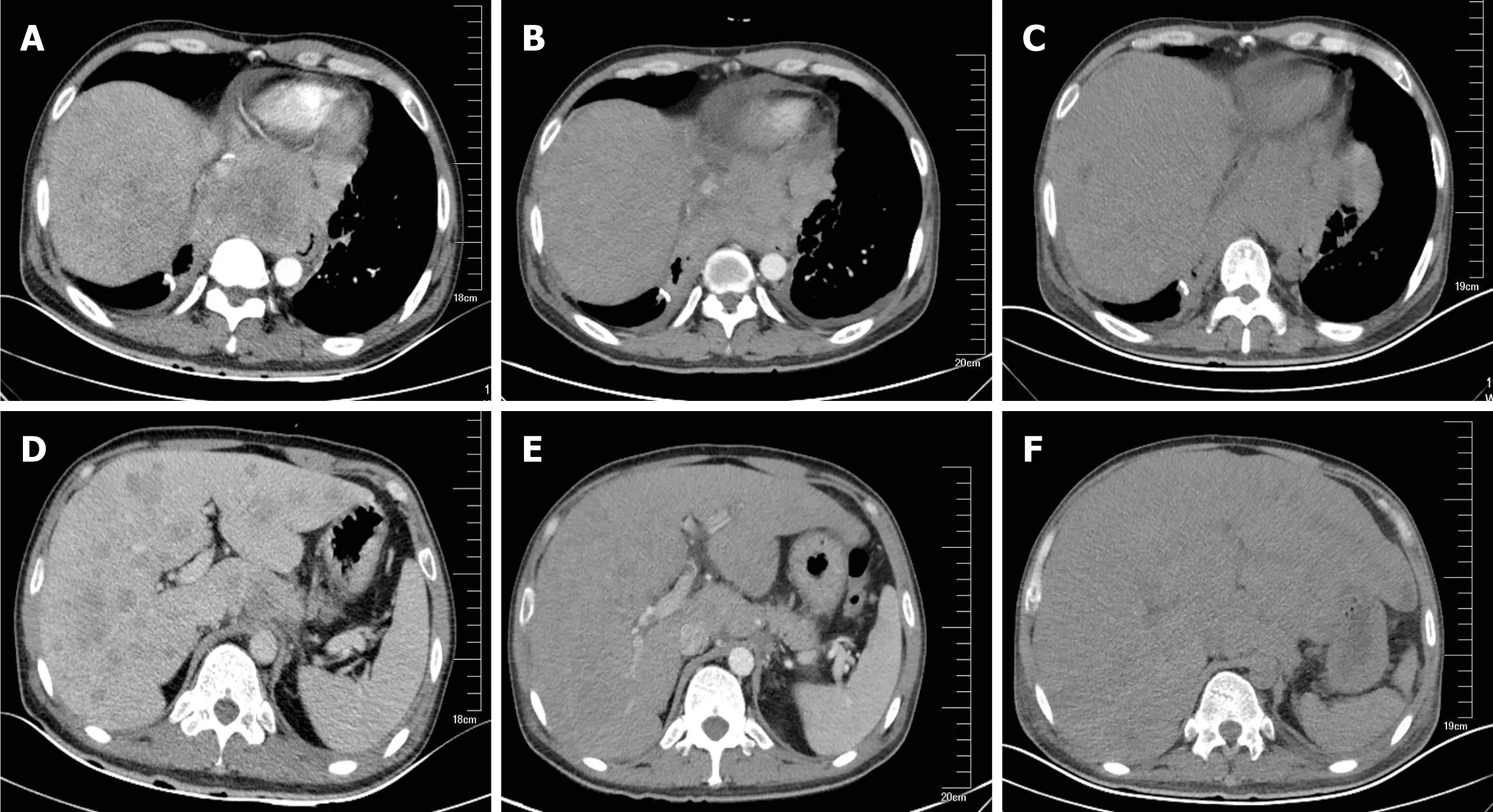
Figure 1 Computed tomography indicated mediastinal and hepatic metastatic changes. A: Chest computed tomography (CT) showed a 8.4 × 9.8 cm mediastinal metastatic lump; B: Chest CT on day 35 showed a slight shrink in mediastinal metastasis; C: Chest CT on day 55 showed unchanged mediastinal lump compared to B; D: Abdominal CT showed multiple metastases; E: Liver metastasis slightly shrank after 35 d of crizotinib therapy; F: Liver volume increased by 20%and acute intrahepatic bile were revealed on day 55.
DISCUSSION
This report describes the first case of fatal crizotinib-induced fulminant liver failure in an Asian patient with liver metastasis. Other causes of liver failure were eliminated,including hepatic metastasis, viral hepatitis infection, biliary obstruction, alcoholic liver disease and concomitant medication. In the beginning, the slightly increased transaminase levels decreased to normal levels after 25 d of oral crizotinib, which indicates that the improvement in liver function was closely related to crizotinib therapy for liver metastasis[17]. However, ALT and AST levels and liver volume suddenly increased on day 55. Although transaminase and total bilirubin levels decreased after two days of crizotinib discontinuation, it increased rapidly again over the next two days, which may have been caused by massive necrosis of liver cells.Based on these findings, crizotinib-induced acute liver injury was strongly suspected.
To better understand the current status of crizotinibinduced fulminant hepatitis, we reviewed and summarized the published literature (Table 1). Four cases of crizotinibinduced hepatitis have been reported, which occurred within a few months after crizotinib treatment. Among these patients, two of them died of hepatic encephalopathy caused by liver failure and one of them died of respiratory failure with carcinomatous pleurisy[18-21]. Consistent with these two cases, the patient in our report also died of crizotinib-induced fulminant liver failure. However, our case was an advanced lung cancer patient with liver metastasis, which is distinct from the reported cases. Thus, early diagnosis and treatment are critical for crizotinib-induced hepatotoxicity, which could helpALK-positive lung cancer patients to obtain survival benefits and avoid fatal events. Weekly liver function tests including transaminases and total bilirubin are insufficient to estimate sporadic crizotinib-induced hepatotoxicity. The King's College Criteria was used to evaluate acute liver failure and poor prognosis. The sensitivity and the specificity of King's College Criteria have been shown to be 68%-69% and 82%-92%, respectively[22]. Therefore, drug-induced liver dysfunction could be diagnosed early and accurately by using the King's College Criteria. Altogether, the King's College Criteria should also be used weekly to evaluate crizotinib-induced acute liver failure.
The mechanism of crizotinib-induced hepatotoxicity remains unclear. Crizotinib is extensively metabolized in the liver byCYP4503A. Consequently, crizotinib andCYP4503A inducers or inhibitors should be avoided at the same time so as not to increase plasma concentrations of crizotinib[23]. Moreover, Yasudaet al[21]have suggested that the underlying mechanism of hepatotoxicity is a partial allergic reaction to crizotinib or its metabolite. Oral desensitization could be considered a viable option after crizotinib-induced hepatitis. In addition, as a novelALKinhibitor,ceritinib could be also used as an alternative agent when crizotinib causes hepatitis[24].Generally, the initial dose of crizotinib was given at 250 mg twice-daily, which does not account for the patient's age, sex, race, body weight, or hepatic function impairment. Therefore, physicians should be aware of serious adverse reactions caused by individual differences[25].
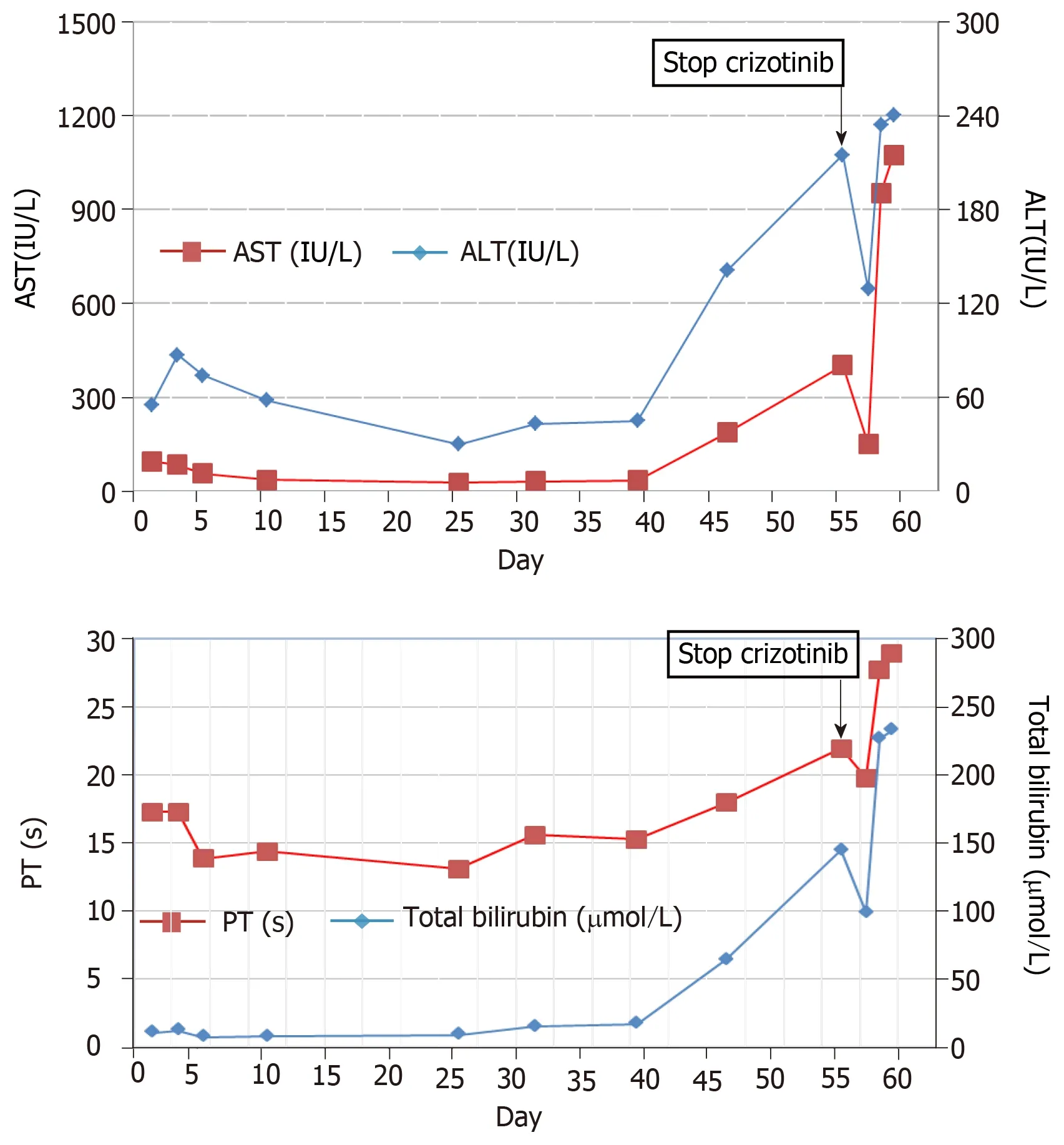
Figure 2 Detailed changes of liver enzymes during crizotinib therapy (day 1-55) and after crizotinib discontinuation (day 56-59). A: Upper graph: aspartate aminotransferase and alanine aminotransferase; B: Lower graph: total bilirubin and prothrombin time. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; PT:Prothrombin time.
CONCLUSION
This study indicates that crizotinib can cause fulminant liver failure in lung cancer patient with liver metastasis. Clinicians should be highly aware of the potential fatal adverse effect induced by crizotinib, especially for patients with liver metastasis. Liver enzyme testing should be carried out at least once a week during the first 2 mo of crizotinib treatment. It is strongly suggested that the King's College Criteria be used to prevent acute liver failure during crizotinib administration in the clinic.
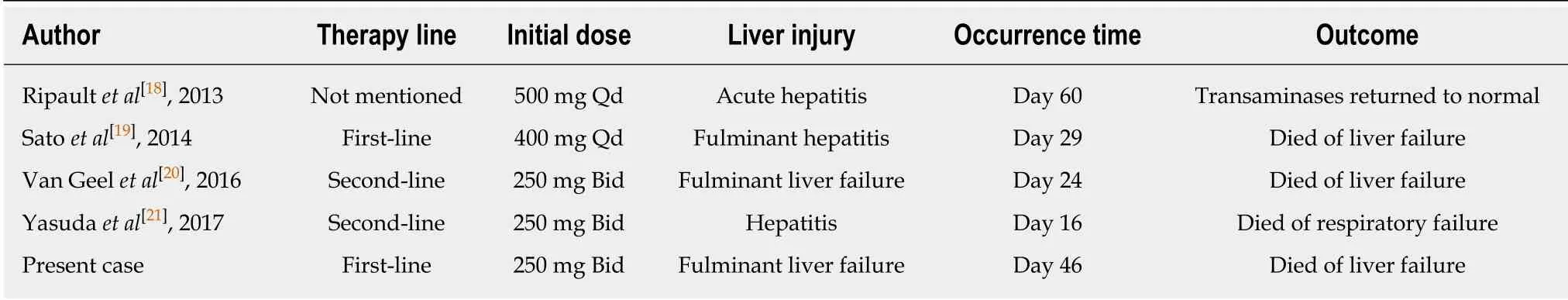
Table 1 Case reports of crizotinib-induced fulminant hepatitis, 2013-2017
 World Journal of Clinical Cases2019年9期
World Journal of Clinical Cases2019年9期
- World Journal of Clinical Cases的其它文章
- Coexistence of breakpoint cluster region-Abelson1 rearrangement and Janus kinase 2 V617F mutation in chronic myeloid leukemia: A case report
- Rare variant of pancreaticobiliary maljunction associated with pancreas divisum in a child diagnosed and treated by endoscopic retrograde cholangiopancreatography: A case report
- Adult-onset mitochondrial encephalopathy in association with the MT-ND3 T10158C mutation exhibits unique characteristics: A case report
- Nerve coblation for treatment of trigeminal neuralgia: A case report
- Management of the late effects of disconnected pancreatic duct syndrome: A case report
- Sofosbuvir/Ribavirin therapy for patients experiencing failure of ombitasvir/paritaprevir/ritonavir + ribavirin therapy: Two cases report and review of literature
