Nano chloroquine delivery against Plasmodium berghei NK65 induced programmed cell death in spleen
Satyajit Tripathy, Sourav Chattopadhyay, Sandeep Kr. Dash, Motlalepula G. Matsabisa,Somenath Roy
1Immunology and Microbiology Laboratory, Department of Human Physiology with Community Health, Vidyasagar University, Midnapore-721102, West Bengal, India
2Department of Pharmacology, University of the Free State, Bloemfontein 9300, Republic of South Africa
3B.S. B. E. Indian Institute of Technology, Kanpur, Uttar Pradesh, India
4University of Gour Banga, Malda, West Bengal, India
Keywords:Splenocyte Apoptosis Nano chloroquine Plasmodium berghei Chitosan
ABSTRACT Objective: To compare the protective effects of chitosan-trypolyphosphate (CS-TPP)nanoparticle conjugated chloroquine(CQ) with effect of CQ alone on the reversal of splenic damages and induction of apoptosis. Methods: Different researches have been carried out to explore the potential role of chitosan based drug delivery system against parasitic diseases.After successive Plasmodium berghei NK65 parasiste infection by intraperitoneal injection in Swiss mice and subsequent parasite development, the ROS generation, anti-apoptotic and pro apoptotic protein levels in spleen were measured. To analyze caspases, flow cytometry study was performed with annexin Ⅴ-FITC and with PI staining. Results: The results revealed that ROS mediated caspase 3 and 9 activation and the induction of apoptosis occurred during the parasitic infection. However, CS-TPP conjugated CQ was relatively better in reversing the splenic damage compared with similar effects of CQ alone. Conclusions: This study indicates that Plasmodium berghei NK65 induces apoptosis in the spleen. The study further shows that CS-TPP nanoparticles conjugation with CQ have positive influence on the recovery of damaged host’s system towards maintenance of normal homeostasis, and this is shown to be selective to CS-TPP conjugated CQ treated animals only.
1. Introduction
Malaria is still prevailing threating from the public health standpoint. The artemisinin combination therapy (ACTs) strategy has been acclaimed by WHO in 2015. But some recent reports regarding reduction of artemisinin combination therapy’s sensitivity by parasites are intimidating this strategy[1,2]. Peculiarly, the parasite is also reported to induce acute injuries to vital organs.The most prominent of these injuries have been found in liver,spleen and kidney of infected hosts. The spleen size has been used as an indicator for the virulence of malaria transmission in endemic regions[3-5]. Several investigations on the causes of organ damage suggest the high free radical generation as well as oxidative stress during malarial infections. During malarial infection development, huge amounts of hemoglobin inside parasite food vacuole are degraded by thePlasmodiumparasite. The degradation of haemoglobin releases a bulky amount of redox active free heme (ferriprotoporphyrin Ⅸ) which is very lethal to both parasite and host[6,7]. There is evidence of liver and spleen dysfunction:histopathological changes including necrosis as a results of the malarial infection[8]. The spleen is a multifaceted, biggest secondary lymphoid organ. This organ is adjusted to specifically filter and eliminates senescent red blood cells as well as infectious microorganisms likePlasmodiumsp. Acute infection by malaria is responsible to cause spleen rupture and splenomegaly.
To restraint and reduce mortalities from malaria, there is an urgent need for new effective safe and quality antimalarial formulations before the full emergence of artemisinin combination therapy resistance. Different studies have been carried out to explore the potential role of chitosan (CS) based antimalarial drug delivery systems to enhance the efficacy of the age-old antimalarial drugs[9-11].Advances in nanotechnology makes it more closer to grasp this goal.It is hypothesized that nanodrug carriers may increase the efficacy of drugs because CS nanoparticles, which contain positive surface charge, may show more affinity to bind with negatively charged infected red blood cells. This particle-conjugated chloroquine (CQ)may bring higher amounts into infected cells than CQ alone. The CS nanoparticle reduces ROS generation in host cell, hence overcomes tissues pathology. With this background, previous reports from our study showed that CS nanoparticle accelerates the delivery of CQ, a 4- aminoquinoline drug. The results further showed that the conjugate reversed and recovered liver damage as well as hepatic and splenic mitochondrial oxidative alteration more significantly than CQ alone treatment[12,13].
The present study is designed to evaluate the degree of damage in spleen caused byPlasmodium berghei(P. berghei) NK65 in Swiss male mice. This study was carried out to find out the possible pathways of programmed cell death in splenocytes and likely role that could be played by CS tripolyphosphate nanoparticle conjugated CQ in reversing organ damage due to plasmodium infections.
2. Materials and methods
2.1. Chemicals and reagents
Sodium chloride (NaCl), ethylene diaminetetraacetate (EDTA),Triton-X 100, potassium hydrogen phosphate (KH2PO4), dipotassium hydrogen phosphate (K2HPO4), sodium hydroxide(NaOH), potassium hydroxide (KOH), diphenylamine were procured from Merck Ltd., SRL Pvt. Ltd., Mumbai, India. Antibiotics, fetal bovine serum were purchased from GIBCO/Invitrogen. RPMI 1640,MTT were purchased from Himedia, India. 5’,5’-dithio (bis)-2-nitrobenzoic acid, reduced glutathione (GSH), NADPH Na4, NADPH were procured from Sigma (St. Louis, MO, USA). CQ diphosphate,CS, dichloro-dihydro-fluorescein diacetate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals used in this study were purchased from Merck Ltd. and Himedia, India, and were of the LC-MS grade available.
2.2. Preparation and characterization of CS-tripolyphosphate nanoparticle (CS-TPP NPs) conjugated CQ
CS-TPP NPs were prepared with CS and sodium tripolyphosphate by ionotropic gelation method[10]. The drug loading with the NPs was confirmed by Fourier transform infrared spectroscopy scanning, size distributions were studied by dynamic light scattering study, surface charge of the particle and drug-loaded particle was measured by Zeta Potential Analyzer from Brookhaven Instruments Corporation and high-resolution transmission electron microscopy was used to observe the morphology of the nanoparticles[10,12].
2.3. Animal and parasites inoculums
Swiss male mice (6-8 weeks old, weight 20-25 g) were used.Animals were kept up in accordance with the guiding principle of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India, and the study was approved by the ethics committee of Vidyasagar University. The animals were provided with standard pellet diet with vitamins, antibiotic and water was givenad libitum. The animals were kept in polypropylene cage(Tarson) with 12 h light and dark cycle under standard temperature[(25±2) ℃] and the mice had no signs of any malignancy or other pathological conditions. By intraperitoneal (i.p.) injection of 200 μL of infected blood containing 1× 105parasites, the mice were infected[14]. The mice were kept separately into same sex and aged wild mice by weekly passage. The blood stage parasites were stored at -80 ℃.
2.4. Experimental design
The randomly selected experimental mice were separated into 4 groups of six mice per group where Group A: Control (not infected and no drug), Group B: Infected control group; Group C: infection +CQ; Group D: infection + nanochloroquine (NCQ). After successive infections development for 10 d in Group B, C, and D, Group A and Group B were treated with normal saline (20 μL) and Group C was treated with 68.4 mg/kg bw CQ by intraperitoneal (27.36%) and Group D was treated with 250 mg/kg bw NCQ by intraperitoneal for 15 d[10]. The animals were dissected and giemsa staining of thick blood smears were observed under microscope for parasitemia counts. The spleens from the mice were excised, washed with cold normal saline and stored in preparation for further studies.
2.5. Parasitemia determination, splenocytes isolation and tissue homogenation
Parasitemia was counted by light microscopy under oil immersion at 100× magnification and measured as a percentage of infected erythrocytes in fields of 1 000 erythrocytes.
Cells from the spleen were isolated from experimental groups according to the method of Seglen[15]. Cell viability was then determined by trypan blue exclusion test and MTT assay.
Tissues were homogenized immediately in the cold buffer containing 0.25 M sucrose, 1 mM EDTA, and 1 mM Tris-Hcl, pH 7.4. The tissue contents were centrifuged at 10 000 rpm for 10 min at 4 ℃. The supernatant was stored at -80 ℃ for later estimation of different biochemical parameters[8]. Bovine serum albumin was used as a standard to quantify the protein according to the method of Lowry et al[16].
2.6. Estimation of malondialdehyde (MDA) and reduced glutathione level in tissue homogenate
Lipid peroxidation was measured in terms of MDA level. Lipid peroxidation of tissue homogenate was estimated by the method of Ohakawaet al[17].
GSH level was estimated in tissue homogenate according to the method of Moronet al[18]. The sample was mixed with 25% of TCA and centrifuged at 2 000×gfor 15 min to precipitate out the proteins.The levels of GSH were expressed as μg of GSH/mg protein.
2.7. Splenocytes staining by 2’,7’-dichlorodihydrofluoroscein diacetate (DCFH2-DA)
To estimate the intracellular ROS levels in isolated cells, DCFH2-DA was used. Samples were incubated in the presence of 10 mM DCFH2-DA in phosphate buffered saline (PBS) pH 7.4 at 37 ℃for 30 min then washed twice with PBS. The cell solution was centrifuged at 1 200 rpm to remove the extracellular DCFH2-DA.The intensity of trapped fluorescent dye at different concentration was monitored spectrophometrically with excitation and emission wavelengths at 498 nm and 530 nm, respectively.
2.8. Flow cytometry analysis by annexin V-FITC/ propidium iodide (PI) double staining
Flow cytometry analysis with annexin Ⅴ-FITC and PI staining were performed to evaluate the splenocytic apoptosis. Firstly,annexin Ⅴ-FITC staining was used following the manufacturer’s specifications (Apoptosis Detection Kit; Sigma-Aldrich); Secondly,PI for nucleus labeling was used to reveal apoptotic nuclei. Briefly,in a hypotonic PI solution [50 μg/mL PI, 0.1% sodium citrate,0.4 mg/mL DNAse-free RNAse, type l-A, 0.1% (vol/vol)] cells were re-suspended and kept in the incubation in the dark for 30 min.Fluorescence was acquired by FACS, and the percentage of cells in the lower right quadrant was considered as early apoptotic and upper-right quadrant was considered as late apoptotic cells[19].
2.9. Enzyme linked immunosorbent assay of apoptotic proteins
Enzyme linked immunosorbent assay was done to determine the apoptotic protein levels as per the methods of Dirks[20]. A 96 well round bottomed plates were coated with 1 μg of protein in a buffer solution (pH 9.8), sealed and left overnight at 4 ℃. The plates were washed with buffer containing PBS with 0.02% sodium azide and 0.05% Tween 20. After that, They were washed by 300 μL of 1% BSA in PBS with 0.02% sodium azide and incubated at room temperature for 60 min in sealed wells. A total of 50 μL of the primary antibody at a concentration of 5 μg/mL diluted with 1% BSA in PBS/azide was incubated for 60 min at ambient room temperature. Each well was washed four times with 50 μL of the secondary antibody at a 1: 2 000 dilution into a solution of 1% BSA in PBS/azide, and the plate was incubated for 60 min at ambient temperature. The washing procedure was then repeated four times, and 100 μL of freshly made substrate solution at a concentration of 1 mg/mL in substrate buffer was added. The plate was then incubated at room temperature for a further 60 min, and the absorbance was read at 405 nm.
2.10. Protein estimation
Splenocytes were centrifuged at 12 000gfor 10 min to estimate the protein as per modifications of Lowryet al[16]. Bovine serum albumin was used as standard to quantify the proteins.
2.11. Statistical analysis
The data has been expressed as mean ± SEM, withn= 6. The means of control and experimental groups were compared by oneway ANOVA test followed by Student’sttest using a statistical package software, Origin 6.1 (Northampton, MA 01060, USA), withP<0.05 as a limit of significance.
3. Results
3.1. Parasitemia and cell viability
Parasitemia was observed in different experimental group. On 25thday, inP. bergheiinfected group parasitemia was 23.19% whereas it was decreased by 67.02% in CQ treated group and decreased by 96.57% in NCQ treated group compared to infected control group.The cell viability assay indicated that there was 92% cell viability after isolation.
3.2. Effect of NCQ particles on lipid peroxidation and GSH level
Lipid peroxidation was estimated by the measuring MDA level.MDA level was increased significantly by 87.40% (P<0.05) in spleen of infected group compared to control. The CQ and NCQ significantly (P<0.05) decreased the MDA level, whereas the decrease in NCQ treated group was 18.49% higher than CQ treated group (Figure 1).
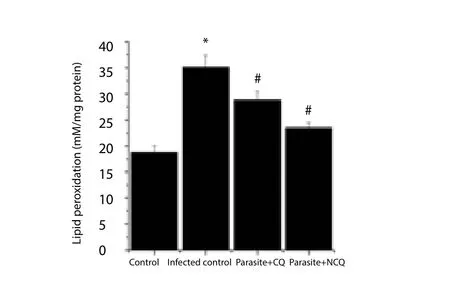
Figure 1. Malonaldehyde levels in spleen of experimental groups.
The results showed that as compared to control, the GSH activity was decreased significantly (P<0.05) in spleen of infected group.CQ and NCQ increased GSH activity compared with infected group.NCQ treated group showed better activity by elevating the GSH,13.3% higher than the CQ only treated group (Figure 2). The low levels of MDA and elevation of GSH levels in NCQ treated group reveals that host cells restored its protection capability.
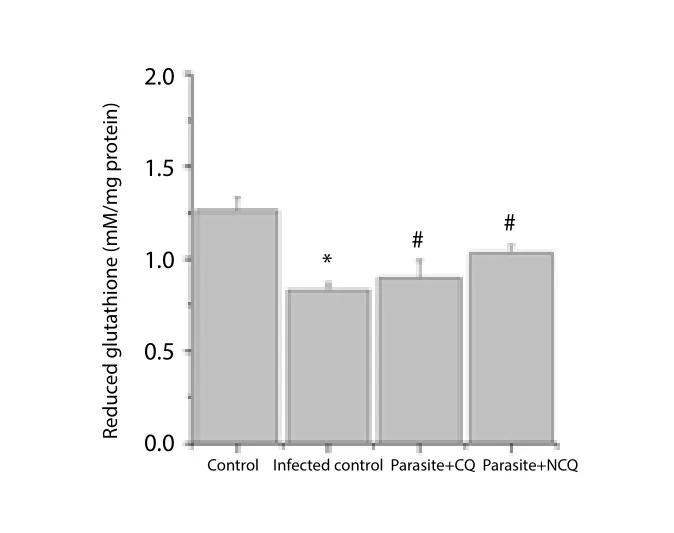
Figure 2. Reduced glutathione level in spleen of experimental groups.
3.3. ROS generation in splenocytes
The results of fluorescence intensity in H2-DCFDA stained splenocytes of experimental groups indicated that parasite infection caused high ROS generation in the cells. The fluorescence intensity of cells infected withP. bergheiwas higher than that of non-infected group; while CQ and NCQ treated group showed lower fluorescence intensity than infected group. The intensity of NCQ treated group was 27.14% lower than CQ treated group (P<0.05) (Figure 3).
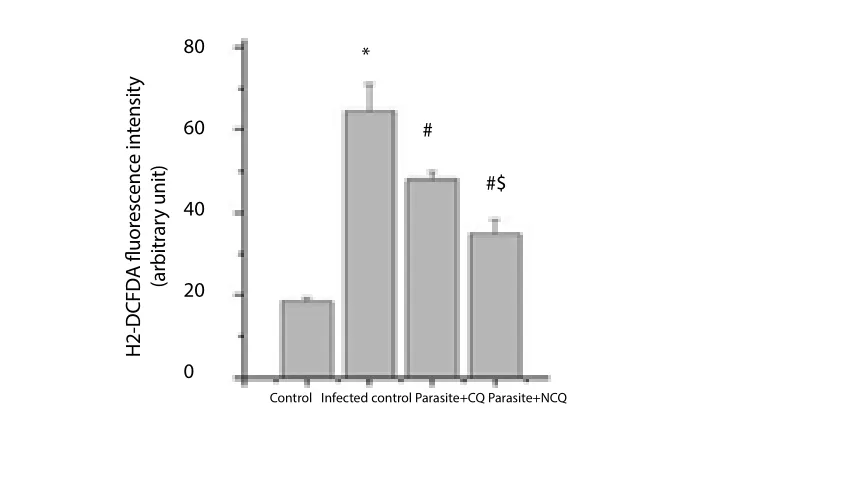
Figure 3. Meanfluorescent intensity in splenocytes.
3.4. Apoptosis
FACS analysis by annexinⅤ-FITC and PI staining in Figure 4 showed that the number of early apoptotic cells (only annexin Ⅴpositive; PI negative) was decreased in CQ treated group by 40.86%and decreased by 69.35%in NCQ treated groups, as compared to infected control group. The late apoptotic splenocytes (both nnexinⅤpositive and PI positive) were decreased in CQ treated group by 34.03% and by 72.57% in NCQ treated groups. The results showed that in the NCQ treated group, the apoptotic cell numbers were lower than infected and CQ treated group.
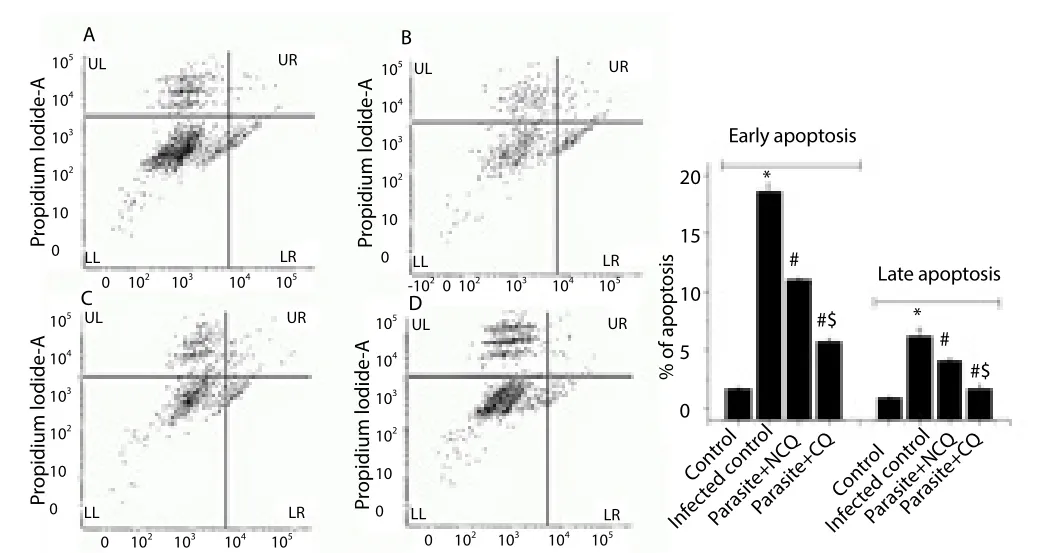
Figure 4. FACS analysis of splenocytes, (A) control, (B) infected control, (C)(parasite + CQ) and (D) (parasite + NCQ) groups.
Lower right (LR) quadrant of each experimental group denotes the percentage of early apoptotic cells (only annexin Ⅴ-FITC positive) and upper left (UL)quadrant of each experimental group denotes the percentage of late apoptotic cells (PI positive, annexin Ⅴ-FITC positive).
Values are expressed as mean ± SEM,n= 6;*Significant difference (P<0.05)compared to control group,#Significant difference (P<0.05) compared to infected group, and$indicates significant difference (P<0.05) compared to only CQ treated group.
3.5. Changes of apoptotic proteins
Pro and anti-apoptotic proteins level like cytochrome C, Bax, Bcl-2,and caspase 3, 8, 9 in splenocytes were measured by enzyme linked immunosorbent assay technique. The results showed that cytochrome C levels was significantly increased by 13.58 fold compared to that of the control group (P<0.05); whereas in CQ treated group, it was decreased by 0.16 fold and decreased by a 0.34 fold in the NCQ treated group as compared to the infected control (Figure 5).
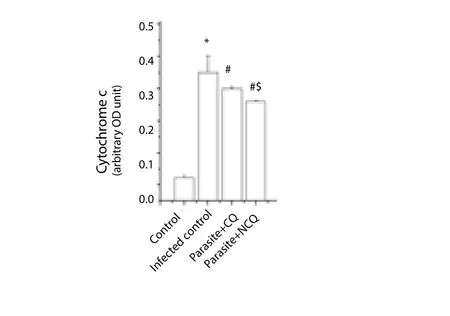
Figure 5. Cytochrome-c level in spleen.
The cytochrome C level increment is also an indication that the organelle is undergoing many apoptotic stimuli and eventually stimulates caspase 9. In this study, caspase 9 was also measured.The result showed that in infected group, caspase 9 was significantly increased (P<0.05) whereas in CQ and NCQ treated group, the level of caspase 3 and 9 were reduced (Figure 6, 7), but difference of caspase 8 levels (Figure 8) were not significant between the infected or treated groups. In this study, pro-apoptotic protein Bax was elevated in infected group (Figure 9) whereas the anti-apoptotic protein, Bcl-2 was decreased in the infected group compared to control group. The CQ and NCQ treated groups showed the significant elevation (P<0.05) of the Bcl-2 relative to infected groups(Figure 10).
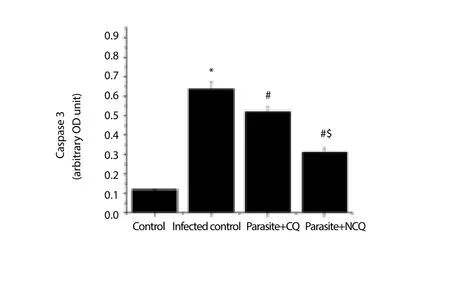
Figure 6. Caspase 3 activity in spleen.
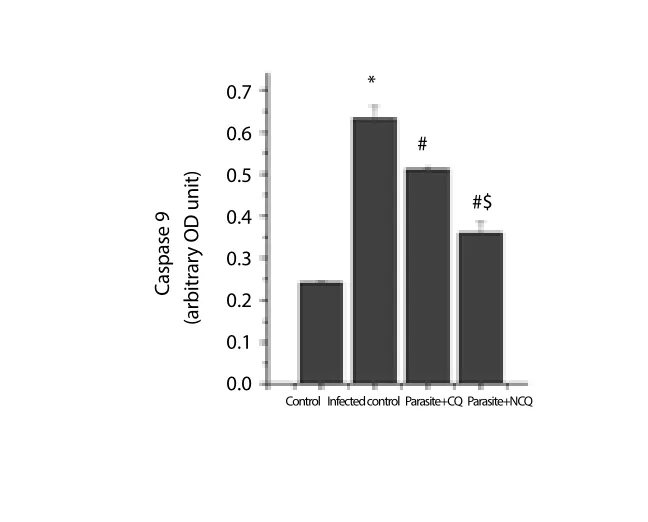
Figure 7. Caspase 9 activity in spleen.

Figure 8. Caspase 8 activity in spleen.
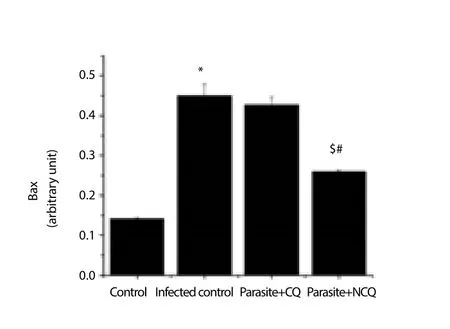
Figure 9. Bax protein level in spleen.
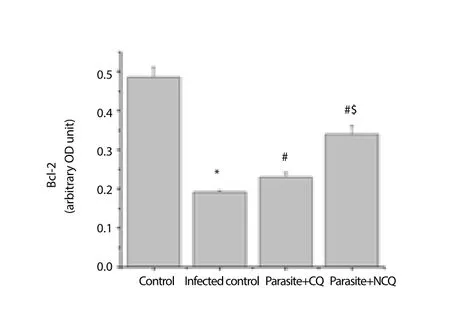
Figure 10. Bcl-2 protein level in spleen.
4. Discussion
To improve pharmacokinetics, effectiveness and safety of drugs,there are different drug delivery process. Recently, CS is regarded as an effective drug delivery vehicle. It helps to re-establish age-old drugs by modifying their pharmacodynamics and pharmacokinetic properties to improve their bio-distribution, bioavailability and reducing toxicity. It has been demonstrated in previous scientific studies that CS-TPP nanoparticles conjugation with CQ increases the efficacy of CQ in reducing virulence and pathological damage byP.bergheiinfection in Swiss male mice. In this study, it has been shown thatP. bergheiinfection induces splenic apoptosisviamitochondria dependent pathway.
CS-TPP conjugated NCQ was synthesized by ionotropic gelation method. The conjugation between the drug and particles, particle size and surface charge, outer surface morphology were characterized and confirmed by Fourier transform infrared spectroscopy, dynamic light scattering, Zeta potential and transmission electron microscopy analysis[10,12]. This CS-TPP conjugated chloroquine particle (NCQ)was applied at its effective dose (250 mg/kg bw) for a duration of 15 days againstP. bergheiNK65 infected mice after successive infection development (after 10 days of infection development)[12].The pathophysiology of malaria depends on the strain ofPlasmodiumspecies and hence the associated antigens involved[21]. The findings of this study revealed thatP. bergheiinfection up-regulate the apoptotic proteins (Bax, caspase 3 and caspase 9) in the splenocytes of wild type mice. Apoptosis is a multifarious cellular process[22].The progression of apoptosis is commenced by various biochemical intermediates, like death factors and reactive oxygen species[23-25].Malaria-induced apoptosis has been reported in monkeys[26] and in humans[27]. The pathogenesis of malaria includes the destructive invasion of erythrocytes, which then induces oxidative stress to the host erythrocytes. The free heme released by parasites during erythrocytic phage contains Fe2+atoms, which can be catalyzed through the Fenton and Haber-Weiss reactions and generates free radicals[28]. It was observed that free radical generation was also increased in splenocytes. In the infected splenocytes, not only ROS level was elevated, but also the lipid peroxidation activity was significantly higher whereas the GSH level decreased than both the control and treated groups. These results indicate the imbalance of oxidative status in the splenocytes in the infected groups, which may leads to release of cytochrome C as well as apoptosis.
The mitochondrial apoptotic pathway plays a critical role in liver cell death during malaria infection[29]. The intrinsic apoptotic pathway is triggered due to cellular stress. These cellular developmental indications lead to liberation of cytochrome c and other pro-apoptotic cofactors from the inter-membrane space of the mitochondria. In this pathway, apoptosis is triggered by the down-regulation of Bcl-2 and Bcl-L and up regulation of pro-apoptotic proteins such as,Bax, Bak[30]. These pro-apoptotic proteins induce pores formation(mitochondrial permeability transition pores) in mitochondria directly and initiate apoptosis[31,32]. The membrane permeabilisation and mitochondria-to-cytosol translocation of cytochrome C activates Bax proteins, which increases mitochondrial membrane permeability,leading to reduce mitochondrial membrane potential. In previous study, it has been found that the mitochondria of liver and spleen are subjected to the oxidative damage[13].
The results further suggest the significant activation of caspase-3 and 9 in the spleen of infected mice, but no significant change was found in caspase 8 expression compared to control mice.Interestingly, the NCQ treated group showed lower expression of caspase 3, 9 than CQ treated group whereas significantly higher elevation of the Bcl-2 level in NCQ treated group has been found compared to infected group. This preliminary data may suggest that CQ alone is less efficient than NCQ. The proteins of Bcl-2 family localize at membrane compartments during apoptosis and regulate the release of cytochrome c from mitochondria. Bcl-2 is an apoptosis suppressing factor that heterodimerizes with Bax and neutralizes the inhibitory effects. When Bcl-2 is present in surplus, cells are sheltered against apoptosis. In contrast, when Bax level is high, cells are vulnerable to apoptosis.
From these results, it is important to note that severe malaria infection causes apoptosis in the host organ due to the excessive oxidative stress, caused by multiplication of parasite in blood and subsequent destruction of red blood cells[12]. CS nanoparticles conjugation with antimalarial drugs could played beneficial role in preventing these processes. This protective role of CS nanoparticle conjugated antimalarial drug, may be linked to the erythrocyte’s membrane. The drug could be possibly internalized in the parasitized red blood cells by passive diffusion and acidic pH dependent.NCQ surface bearing positive charge helps to increase intracellular antimalarial drug, which results in parasites killing, resultant, and reduction of oxidative stress in the host and protection of the organs from severe damage as well as apoptosis.
In conclusion,P. bergheiNK65 infection in some way is capable of suppressing the mode of action of pro-apoptotic protein Bcl-2.Bcl-2 abrogates the pathological effect mediated by Bax. This is possibly through a mitochondrial, caspase 9 and caspase 3 mediated splenocyte apoptosis. Our results further confirm that application of CS nanoparticle conjugated CQ has more efficaciousness in the reduction of parasitaemia as well as the reduction of the generation of reactive oxygen species than only CQ. It is also important to note from these results that malaria infection causes apoptosis in the host organ due to the mitochondrial damage in cellular system, caused by multiplication of parasite in blood. The CS nanoparticles conjugation with antimalarial drug reverses this mitochondrial damage by increasing the efficacy of the drugs. This strategy of antimalaria drug delivery may become the future research and development focus for new therapeutic malaria treatment options. However, further studies are still need to explain the mechanism of action, and to explore clinical application of the CS nanoparticle and formulation of these conjugates in the development of safe, effective and cost effective antimalarial drugs.
Conflict of interest statement
The authors declare that they have not any form of conflicts of interest in the conduct of this study.-
Acknowledgement
The authors express gratitude to the Indian Institute of Technology,Kharagpur, India, Vidyasagar University, Midnapore, India and University of the Free State, Bloemfontein, South Africa and the authors also acknowledge support from the IKS-based Technology Innovation, Department of Science and Technology, South Africa for providing the facilities to execute these study.
 Asian Pacific Journal of Tropical Medicine2018年9期
Asian Pacific Journal of Tropical Medicine2018年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Acute and chronic toxicity studies of hydromethanol leaf extract of Helianthus annuus Linn. in rats
- Distribution and ecological aspects of sand flies (Diptera: Psychodidae)species in Northeastern Iran
- Ameliorative effect of Pergularia daemia (Forssk.) Chiov. leaves extract against anti-tuberculosis drugs induced liver injury in rats
- Antihyperglycemic effect of Passiflora glandulosa cav. fruit rinds flour in streptozotocin-induced diabetic mice
- Role of dietary phytochemicals in modulation of miRNA expression:Natural swords combating breast cancer
- Enhanced surveillance and cohesive policies needed to tackle Rift Valley fever
