Particular evolution in a 72-year-old diabetic patient with acute coronary syndrome
Gabriela S Gheorghe, Ana Ciobanu,*, Ioan T Nanea, Andreea S ?erban, Mihaela R Mititelu
?
Particular evolution in a 72-year-old diabetic patient with acute coronary syndrome
Gabriela S Gheorghe1, Ana Ciobanu1,*, Ioan T Nanea1, Andreea S ?erban1, Mihaela R Mititelu2
1Department of Internal Medicine and Cardiology, “Theodor Burghele” Clinical Hospital, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania2Nuclear Medicine Department, “Dr. Carol Davila” Central Emergency Military Clinical Hospital, Bucharest, Romania
J Geriatr Cardiol 2018; 15: 513?516. doi:10.11909/j.issn.1671-5411.2018.07.008
Diabetes; Elderly; Myocardial infarction
Diabetes mellitus is an important risk factor for coronary atherosclerosis and many patients present extensive coronary stenosis at coronarography. However, in patients with diabetes, endothelial and microvascular dysfunction also participate in chronic and acute myocardial ischemia. Although the majority of diabetic patients with myocardial infarction have angiographic evidence of significant coronary artery disease, they can also experience myocardial infarctions with non-obstructive coronary arteries (MINOCA).[1]The mechanisms leading to myocardial necrosis in these cases are heterogeneous and patients often present atypical evolution, making the diagnostic process and the choice of optimal treatment difficult.
A 72 years old woman was admitted to our hospital for progressive dyspnoea and one episode of haemoptysis occurring one week prior to admission. She affirmed long- term moderate effort retrosternal pain, with episodes lasting about 15 min and resolving with rest. Her medical history included type II diabetes mellitus for 15 years and arterial hypertension, treated with metformin, irbesartan, indapa-mide, acetyl salicylic acid. She had also untreated dyslipidaemia.
The clinical examination revealed a 29.3 kg/m2body mass index, a respiratory rate of 20 respirations per min, 36.8 °C, a spontaneous oxygen saturation of 93%, blood pres-sure of 145/70 mmHg, heart rate 90 per min, left ventricular protodiastolic gallop, grade II holosystolic murmur at the apex, right-sided moderate pleural effusion, jugular veins distention, tender hepatomegaly and discrete legs oedema.
Laboratory tests showed mild normochrome, normocytic anaemia (hemoglobin 11 g/dL), hyperglycaemia (255 mg/dL), glycated hemoglobin of 11.4 %, normal renal function (se-rum creatinine 0.9 mg/dL with and estimated glo-merular filtration rate of 64 mL/min/1.73 m2), inflammatory syn-drome (erythrocyte sedimentation rate of 53 mm/1 h, C re-active protein 40 mg/dL).
ECG showed sinus tachycardia, atrial premature beats, slow R wave progression in precordial leads, ST segment elevation in leads V1-V5, with a maximum of 2 mm in lead V2 and biphasic T waves V4-V6 (Figure 1).
Cardiac markers of necrosis were slightly elevated: creatine-kinase-MB (CKMB) level of 31 UI/L (normal limit < 25 UI/mL) and troponin T (TnT) of 0.02 ng/mL (normal values < 0.01 ng/mL).
The pleural effusion had biochemical characteristics of transudate.
The thoracic radiography showed a right inferior lobe pneumonic process and a small pleural effusion. The thoracic computer tomography scan confirmed these data and excluded pulmonary embolism.
Transthoracic echocardiography showed an undilated left ventricle, moderate left ventricular concentric hypertrophy, akinetic interventricular septum, severe global left ventricular dysfunction [left ventricular ejection fraction (LVEF) of 35%] and elevated left ventricular filling pressures, moderate mitral regurgitation, moderate functional tricuspid regurgitation and high probability of pulmonary hypertension (estimated systolic pulmonary artery pressure of 65 mmHg).
Our diagnosis was ST segment elevation acute myocardial infarction, congestive heart failure, right lower lobe pneumonia, arterial hypertension, uncontrolled type II diabetes mellitus, dyslipidemia.
The patient was treated with ticagrelor 180 mg, acetylsalicylic acid 375 mg, enoxaparin 80 mg, metoprolol succinate 50 mg, atorvastatin 80 mg and referred to the Cath Lab. Coronary angiography revealed a 30% left anterior descending artery (LAD) stenosis, without other anatomically significant lesions. During the procedure the patient devel- oped a significant LAD coronary spasm which re-solved with intracoronary nitroglycerine.
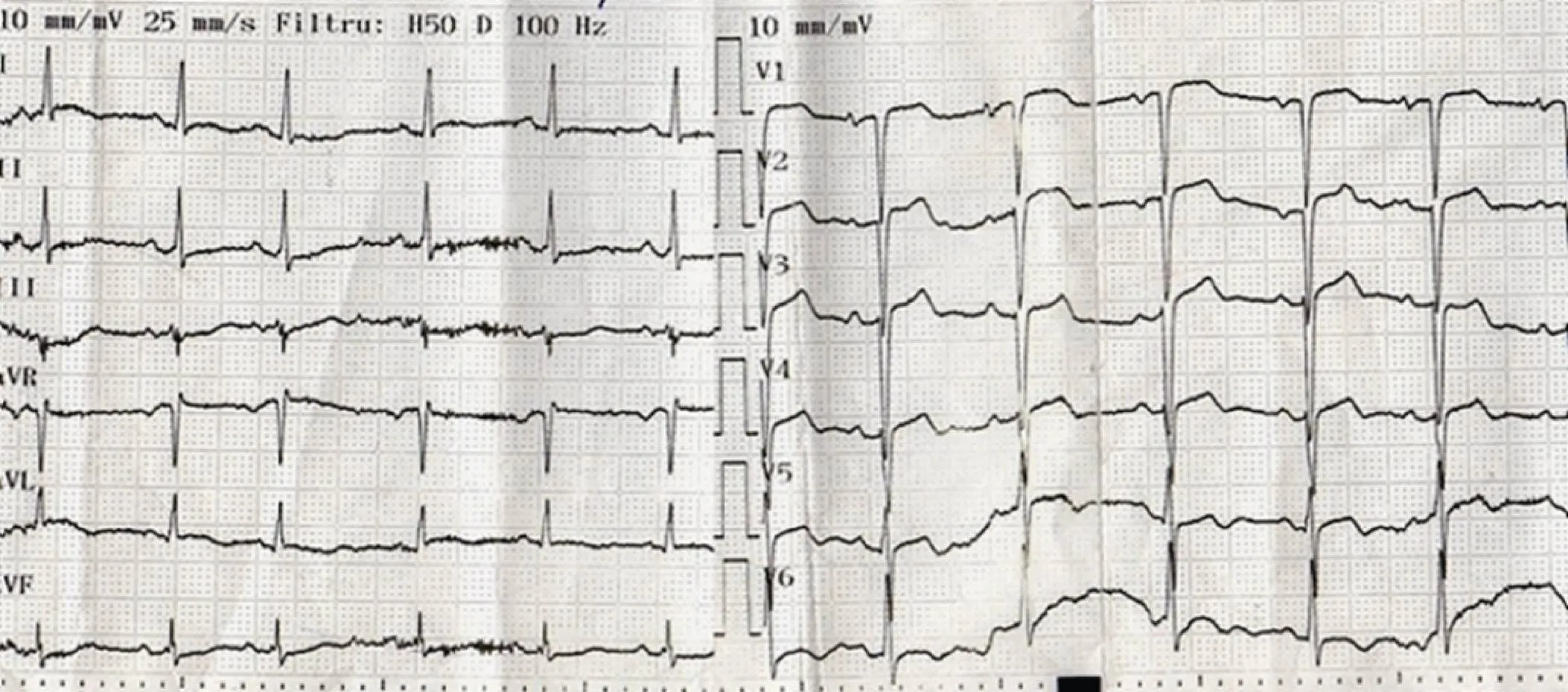
Figure 1. ECG at admission. Sinus tachycardia, QRS axis at 30°, atrial premature beats, ST segment elevation in leads V1-V5, with a maximum of 2 mm in lead V2 and biphasic T waves V4-V6, discrete horizontal ST segment depression in leads DII, DIII and aVF.
Figure 2. ECG evolution after 30 days from admission. The ST segment elevation resolves, T waves become negative in the precordial leads and positive in aVR, QTc interval prolongs significantly, up to 560 ms.
The symptoms improved in the next days and the values of CKMB, TnT normalized. LVEF increased to 45% in a week.
ECG showed ST elevation resolution, prolongation of the QTc interval up to 560 ms, with positive T wave in aVR and symmetrical deep negative T waves in the precordial leads and DI, DII, aVL (Figure 2).
We performed myocardial scintigraphy at rest (ECG gated SPECT) which showed discrete hypoperfusion in the inferior wall and the interventricular septum, associated with hypokinesis, but with preserved systolic thickening and a normal global left ventricular ejection fraction (Figure 3).
During the 10-th day after her admission the patient pre-sented dizziness and her heart rate was 36/min. ECG sub-sequently showed sinus bradycardia, sinus pauses, isorhy-thmic atrio-ventricular dissociation, junctional escape rhy-thm (Figure 4).
The laboratory tests, including serum potassium and thyroid hormones, were normal.

Figure 3. ECG gated SPECT at rest: discrete inferoseptal and inferior hypoperfusion, inferior and septal hypokinesis, with preserved systolic thickening in these territories and a normal global left ventricular ejection fraction. SPECT: single photon emission computed tomography.
Figure 4. ECG evidence of rhythm disturbances, starting day 10. First strip: sinus pause of 3.7 s. Second strip: narrow QRS complexes with a rate of 43/min; the first three QRS complexes are not preceded by P waves; from the 4thQRS complex atrio-ventricular dissociation dissociation is observed, with P waves at a rate of about 22/min, suggesting atrio-ventricular dissociation with junctional escape rhythm. Then the ECG shows sinus bradycardia (the last two P waves seem to be transmitted, with a PR segment of 0,16 s).
We assumed that the beta blockers revealed a previous latent sinus node dysfunction. After beta blockers were stopped, the conduction disturbances did not improve and the patient received a DDDR pacemaker.
Our patient had many particularities. Firstly, diabetes is known to be associated with an increased risk of silent acute coronary syndromes. Often angiography shows multivascular coronary severe stenosis. However our patient had non-obstructive coronary arteries despite her longstanding history of poorly controlled diabetes. She had instead severe but reversible coronary spasm, which could have been responsible for the myocardial ischemia. On the other hand, microvascular coronary disease and endothelial dysfunction demonstrated a strong association with diabetes mellitusand are also involved in the occurrence of acute coronary syndromes.[2,3]Unfortunately, we did not perform fractional flow reserve and coronary flow reserve. The evaluation of the endothelial function by acetylcholine is not validated in patients with diabetes and acute coronary syndromes.[3]
Secondly, our patients had concomitant pneumonia. Systemic inflammation can activate the coronary atheroma plaques and also can produces type 2 myocardial infarction by altering the supply-demand balance. The incidence of type 2 myocardial infarction in a large cohort study was 7.3% in patients with myocardial infarction.[4]But among patients with non-significant coronary artery stenosis, a profound supply-demand mismatch is needed to determine acute myocardial infarction.[1]In addition, 97% of type 2 myocardial infarctions present with ECG changes of non-ST segment elevation myocardial infarction.[5]
Our patient had a particular ECG evolution, with sinus bradycardia, sinus pauses, and junctional escape rhythm. There is no clear explanation of these modifications in the absence of extracardiac causes and without angiographic evidence of significant lesions of the coronary arteries. Progressive ischemia due to endothelial and microvascular dysfunction could be an explanation, but these disturbances occurred late in the evolution, when the laboratory inflammatory and cardiac necrosis tests already normalized. We believe that the beta blocker treatment unmasked a latent sinus node dysfunction. The hypothesis of myocarditis can also be raised, but the cardiac scintigraphy showed regional perfusion defects associated with abnormal kinetics in the same territories.
Also, the evolution of the T waves which became negative, deep, symmetrical in epicardial leads and positive in aVR suggest multivascular coronary involvement, though this was not confirmed by the coronarography.
We cannot completely remove the hypothesis of a myocarditis despite the myocardial scintigraphy describing regional perfusion and wall motion defects, cardiac magnetic resonance imaging being the standard for this diagnosis. Despite our efforts with the purpose of a correct and complete diagnosis, the patient refused to continue the investigations.
Our final diagnosis was MINOCA due to coronary spasm, reproducible during the coronarography, but probably also to microvascular coronary dysfunction, in an uncontrolled diabetic and dyslipidemic patient, with acute pneumonia. The conduction disturbancies needing permanent cardiac pacing seemed to be due to a latent sinus node dysfunction unmasked by administration of beta blockers. MINOCA has several possible etiologies, sometimes atypical evolution and poses many challenges to the practitioner.
1 Agewall S, Beltrame JF, Reynolds HR,. ESC working group position paper on myocardial infarction with non-ob-structive coronary arteries.2017; 38: 143–153.
2 Picchi A, Capobianco S, Qiu T,. Coronary microvascular dysfunction in diabetes mellitus: a review.2010; 2: 377.
3 Kibel A, Selthofer-Relatic K, Drenjancevic I,. Coronary microvascular dysfunction in diabetes mellitus.2017; 45: 1901–1929.
4 Radovanovic D, Pilgrim T, Seifert B,. Type 2 myocardial infarction: incidence, presentation, treatment and outcome in routine clinical practice.2017; 18: 341–347.
5 Saaby L, Poulsen TS, Hosbond S,. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction.2013; 126: 789–797.
Correspondence to: ana_ciobanu@outlook.com & ana.ciobanu@umfcd.ro
 Journal of Geriatric Cardiology2018年7期
Journal of Geriatric Cardiology2018年7期
- Journal of Geriatric Cardiology的其它文章
- Single-territory incomplete surgical revascularization improves regional wall motion of remote ventricular areas: results from a propensity-matched study
- A three-year longitudinal study of the relation between left atrial diameter remodeling and atrial fibrillation ablation outcome
- Management of hypertensive crises in the elderly
- C-reactive protein aggravates myocardial ischemia/reperfusion injury through activation of extracellular-signal-regulated kinase 1/2
- Prognostic utility of NT-proBNP greater than 70,000 pg/mL in patients with end stage renal disease
- The effectiveness and safety of the RESTORE? drug-eluting balloon versus a drug-eluting stent for small coronary vessel disease: study protocol for a multi-center, randomized, controlled trial
