A novel natural compound Shikonin inhibits YAP function by activating AMPK
Fang-Jie Yan, Mei-Jia Qian, Hong Luo, Chen-Ming Zeng, Tao Yuan, Qiao-Jun He, Hong Zhu*, Bo Yang*
1Zhejiang Province Key Laboratory of Anti-Cancer Drug Research, College of Pharmaceutical Science, Zhejiang University, Hangzhou, China. 2Zhejiang cancer hospital, Hangzhou, China.
Background
Cancer is the leading cause of diseases-related death, with globally increasing numbers of cases and death.Therefore, the exploration of new and effective anti-cancer drugs is urgently needed. Analyzing the sources of newly approved drugs for the treatment of human diseases, natural products play noteworthy significant roles in the drug discovery and development process [1,2]. A large number of compounds originated from natural products have exhibited highly effective functions in cancer therapies, these natural derived anti-cancer drugs including Taxol, vincristine and camptothecin [3,4]. However, with the vigorous development of molecular targeting therapies, yet the drug discovery from natural products have diminished in the past two decades, for natural products were frequently used for their medicinal properties but barely investigated the role at molecular level [5,6]. Using specific models,natural products are promising to be applied to drug discovery approaches which are based on high-throughput screening (HTS) directed at molecular targets.
Hippo pathway is highly conversed in mammals, with the function to regulate cell proliferation, apoptosis and stemness [7]. The upstream regulators of core kinase cascade include MST1/2 and LATS1/2. The downstream effector of Hippo pathway is YAP (Yes-Associated Protein) that is negatively regulated by MST1/2 and LATS1/2. YAP functions as a transcriptional co-activator and regulates the transactivation of a variety of target genes. Overexpression of YAP results in cell transformation and tumour development, indicating that YAP could be a promising target of cancer treatment.Recently, much effort has been taken to explore potential inhibitors of YAP. Through an established luciferase reporter assay named Gal-TEAD4 which stimulated by YAP, more than 3300 drugs were collected to be screened[8], and Verteporfin was identified as the top of hits [9],yet the anti-cancer efficacy remained to be improved for this compound. As the source of natural products is huge and yet to be explored, high-throughput screen from natural products has entered into our sight. Here we use a luciferase reporter containing eight copies of the TEAD DNA-binding sequence (8×GTIIC) to identify the natural compounds that suppress the transcriptional activity of YAP [10]. Our study explored a new small molecular inhibitor of YAP, Shikonin, which was presenting in the root tissues of the traditional Chinese medical herb Zicao(Lithospermum erythrorhizon) and investigated the anti-cancer activity of this compound [11].
Methods
8×GTIIC luciferase reporter assay
The 8×GTIIC luciferase reporter contains eight copies of the TEAD DNA-binding sequence to investigate the transcriptional activity of YAP. The 8×GTIIC luciferase reporter plasmid was purchased from Addgene (Catalog: 34615). The other luciferase reporter Renilla was used as an internal control. Luciferase Reporter Assay Kit was purchased from Promega
Immunoblotting analysis
Immunoblotting was performed using a standard protocol.The information of primary antibodies shows as follows:phosphor-YAP (S127) (#4911, 1:1000), YAP (#4912,1:1000), phosphor-AMPKα (T172) (#2535L, 1:1000) and AMPKα (#5832, 1:1000) were obtained from Cell Signaling Technology. Beta-Actin (db10001, 1:3000) was purchased from Diagbio.
RNA isolation and real-time PCR
Total RNA was extracted with the EasyPure RNA kit(Transgen Biotech, Being, China). RNA then was used for reverse transcription with Transcript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (Transgen).cDNA was diluted and used for real-time with SYBR?-Green (Bio-Rad). The cDNA pools were amplified in a 96-Well Thermal Cycler (Applied Biosystems). And primers were described in (Biosune,Shanghai, China);

Sulforhodamine B (SRB) assay
Cells were seeded on 96-well plates. After incubation with gradient concentration of Shikonin (dissolution in DMSO) for 24h, cell viability was assessed by SRB assay and cell proliferation rate was calculated as A510treatedcells/A510controlcells× 100% [12].
Statistical analysis
Each experiment was repeated three times as mentioned in each figure legend. Data were presented as the mean±s.e.m. The data were analyzed by using SigmaPlot 10.0.
Results
Identification of shikonin as a natural inhibitor of YAP
In order to screen natural inhibitor(s) of YAP from a library containing 70 natural compounds, we established a cell-based YAP inhibitor screening model in human HCC cell line HepG2 by co-expressing 8×GTIIC-luciferase reporter with constitutively active CMV-Renilla luciferase reporter (served as internal control). Figure 1A showed that most natural compounds imposed minimal inhibitory effects against YAP function(or even activated it); while among them, Shikonin(Figure 1B) shown the strongest inhibitory effect on YAP-TEADs luciferase activity, which can significantly reduce the YAP-TEADs luciferase activity (inhibition ratio=74.3%) (Figure 1C). Thus, we were encouraged to speculate that Shikonin might be a natural derived inhibitor of YAP function.

Figure 1. Identification of Shikonin as a natural inhibitor of YAP.
Shikonin suppressed YAP signaling by induced the phosphorylation of YAP
The aforementioned data suggested that Shikonin might be a potent YAP inhibitor, thus we were inspired to evaluate whether this compound was able to interfere with the YAP signaling. Since the phosphorylation at S127 of YAP would lead to the cytoplasmic retention and subsequent proteasomal degradation, which prevents the nuclear translocation and functional activation, the phosphorylated YAP is generally regarded as an inactivated form of YAP. As shown in Figure 2A,Shikonin treatment for 24 h could elevate the phosphorylation of YAP; as a known inhibitor on YAP activity, Atorvastatin, a HMGCR inhibitor was introduced as positive control [13,14]. Next, we sought out to examine the YAP-inhibitory effect upon short-exposure of Shikonin (1 h, 3 h and 6 h, respectively). Intriguingly, 1 h-treatment of Shikonin led to robust upregulation of p-YAP in HepG2 cells, which was even more significant than that in 3 h-and 6 h-groups (Figure 2B); similar observation was achieved in HeLa cells (Figure 2C).These data indicated that Shikonin exerted the inhibitory effect against YAP in a rapid manner.
In order to further validate the effect of Shikonin on YAP function, we analyzed the mRNA levels of YAP target genes including CTGF and AREG. Figure 2D showed that mRNA levels of these two genes were obviously reduced by Shikonin. These data confirmed the initial screening results and indicated that Shikonin interfered with YAP signaling by increasing the YAP phosphorylation and reducing target genes transactivation.
AMPK activation by Shikonin may contribute to the inhibitory effect on YAP
Previous studies have revealed that the activation of AMPK can lead to the suppression of YAP expression[15,16]. Thus, we speculated that the effect of Shikonin on YAP inhibition in HCC cells might be mediated by AMPK signaling. To test this hypothesis, we first verified the inhibition of YAP by activation of AMPK using an energy stress model. As expected, glucose starvation activated AMPK signaling by phosphorylation in HepG2 cells, accompanied with an increased YAP phosphorylation which denoted the blockade of YAP pathway (Figure 3A). Then we asked whether Shikonin could affect AMPK signaling. As expected, the phosphorylation level of AMPK at HepG2 was significantly increased in response to Shikonin treatment in a dose-dependent manner (Figure 3B).
We also compared Shikonin with Atorvastatin,which was able to suppress YAP activity [17].Intriguingly, Atorvastatin elevated the YAP phosphorylation while imposing little effect on AMPK,indicating that Atorvastatin-mediated YAP inhibition was not attributable to AMPK signaling (Figure 3C). Taken together, these data implicated that Shikonin may function as a YAP inhibitor with different mechanism of action of Atorvastatin; which is AMPK signaling might be involved in Shikonin-caused YAP suppression in HCC cells.

Figure 2. Shikonin suppressed YAP signaling by induced the phosphorylation of YAP.
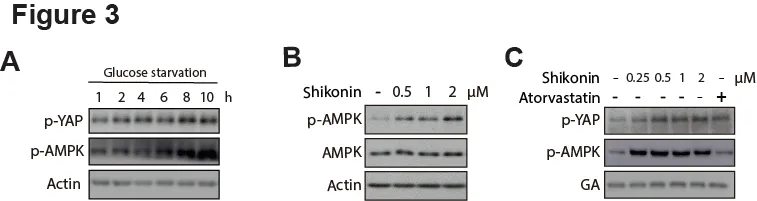
Figure 3. AMPK activation by Shikonin may contribute to the YAP-inhibition.
Shikonin exerted anti-cancer activities against HCC cells
Aforementioned data showed that Shikonin inhibited YAP through AMPK activation. Since mounting evidences have revealed that YAP is an essential modulator of cell proliferation and apoptosis, in addition, YAP is highly activated in HCC models. Thus, the effect of Shikonin on the proliferation of HCC cells was then evaluated by SRB assay. HepG2 cells were exposed to Shikonin at 0, 2.5, 5,10 and 20 μM for 24 h. As showed in Figure 4A, the viability of HepG2 was significantly inhibited by Shikonin in a concentration-dependent manner.Meanwhile, morphological changes in Shikonin-treated HepG2 cells were also observed (Figure 4B). Together with the above-described findings, we were encouraged to speculate that Shikonin may inhibit YAP induced overgrowth in vitro, providing a new insight into HCC therapy using YAP-targeting natural compounds.

Figure 4. The anti-cancer activities of Shikonin in liver cancer cells.
Discussion
YAP overactivation is highly correlated with poor prognosis of cancer patients and has been regarded as a potential target for cancer therapy. However, it still has been challenged by the lack of effective inhibitors against YAP activity, particularly those origins from natural resource. Verteporfin, a phototsensitizer in photodynamic therapy, functioned as an inhibitor disrupting YAP-TEAD complex, yet the anti-cancer of Verteporfin remained to be proved. Meanwhile, limited evidence has revealed the natural compound could suppress YAP function so as to exert anti-cancer activities. Therefore, our findings that Shikonin, a natural naphthpquinone isolated from traditional Chinese medicine, which exhibited an effective inhibition of proliferation on cancer cells, may help fill up the gap of inhibitor scarcity.
The anticancer effect of Shikonin has been demonstrated in a variety of cancers via activation of different signaling pathways. It has been shown to sacrifice leukemia cells through inhibition of c-MYC and a mechanism involving of ERK/JNK/MAPK and AKT pathways [18]. Zhang et al. also demonstrated that it could inhibit the migration of glioblastoma cells by targeting phosphorylated β-Catenin and phosphorylated PI3K/Akt [19]. The antitumor effect of Shikonin mediated by SIRT2 and p-ERK signaling in colon cancer were also emphasized [20]. Meanwhile, Shikonin could enhance sensitization of Gefitinib against non-small cell lung cancer via inhibition of PKM2/stat3/cyclinD1 signal pathway [21].
However, few of the mechanism above mentioned the effect of YAP, if any, the phosphorylation AMPK induced has not been elucidated. Karel et al. revealed
that activation of MST1 by Shikonin inhibited the expression of GLUT1 and C-MYC via the MST1-YAP1-TEAD1 axis [22]. The results also confirm an increase in YAP1 phosphorylation following Shikonin treatment, which is consistent with the observation achieved from our current study. However, our study provides a new mechanism of action by which Shikonin suppressed YAP activities, which was probably owing to the activation of AMPK and the subsequent rapid inhibition on YAP function.
Conclusion
Shikonin exhibited a distinct effect on inhibition of YAP activity by enhancing the upstream of YAP, AMPK kinase activity and suppressing HCC cells proliferation.Collectively, these results implicated that Shikonin might be a promising drug candidate for the treatment of HCC and merit further clinical investigation.
1. Newman DJ, Cragg GM, Snader KM. Natural Products as Sources of New Drugs over the Period 1981?2002. J Nat Prod 2003, 66: 1022-1037.
2. Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod 2016, 79: 629-661.
3. Blajeski AL, Phan VA, Kottke TJ, et al. G1 and G2 cell-cycle arrest following microtubule depolymerization in human breast cancer cells. J Clin Investig 2002,110: 91-99.
4. Beretta GL, Gatti L, Perego P, et al. Camptothecin Resistance in Cancer: Insights into the Molecular Mechanisms of a DNA-Damaging Drug. Curr Med Chem 2013, 20: 1541-1565.
5. Haque I, Subramanian A, Huang CH, et al. The Role of Compounds Derived from Natural Supplement as Anticancer Agents in Renal Cell Carcinoma: A Review. Int J Mol Sci 2017, 19.
6. Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 2015, 14:111-129.
7. Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163: 811-828.
8. Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control.Genes Dev 2008, 22: 1962-1971.
9. Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP.Genes Dev 2012, 26: 1300-1305.
10. Thanh Nguyen H, Andrejeva D, Gupta R, et al.Deubiquitylating enzyme USP9x regulates hippo pathway activity by controlling angiomotin protein turnover. Cell Dis 2016, 2: 16001.
11. Mao X, Rong Yu C, Hua Li W, Xin Li W. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res 2008, 18: 879-888.
12. Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006, 1: 1112-1116.
13. Santinon G, Pocaterra A, Dupont S. Control of YAP/TAZ Activity by Metabolic and Nutrient-Sensing Pathways. Trends Cell Biol 2016,26: 289-299.
14. Sorrentino G, Ruggeri N, Specchia V, et al.Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 2014, 16:357-366.
15. Mo JS, Meng Z, Kim YC, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol 2015, 17:500-10.
16. DeRan M, Yang J, Shen CH, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep 2014, 9: 495-503.
17. Zhou TY, Zhuang LH, Hu Y, et al. Inactivation of hypoxia-induced YAP by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells. Sci Rep 2016, 6: 30483.
18. Zhao Q, Assimopoulou AN, Klauck SM, et al.Inhibition of c-MYC with involvement of ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and its derivatives in killing leukemia cells. Oncotarget 2015, 6: 38934-38951.
19. Zhang F-Y, Hu Y, Que Z-Y, et al. Shikonin Inhibits the Migration and Invasion of Human Glioblastoma Cells by Targeting Phosphorylated β-Catenin and Phosphorylated PI3K/Akt: A Potential Mechanism for the Anti-Glioma Efficacy of a Traditional Chinese Herbal Medicine. Int J Mol Sci 2015 16:23823-23848.
20. Zhang L-L, Zhan L, Jin Y-D, et al. SIRT2 mediated antitumor effects of shikonin on metastatic colorectal cancer. Eur J Pharmacol 2017, 797: 1-8.
21. Tang J-c, Ren Y-G, Zhao J, et al. Shikonin enhances sensitization of gefitinib against wild-type EGFR non-small cell lung cancer via inhibition PKM2/stat3/cyclinD1 signal pathway. Life Sci 2018 204: 71-77.
22. Vali? K, Talacko P, Grobárová V, ?erny J, Novák P.Shikonin regulates C-MYC and GLUT1 expression through the MST1-YAP1-TEAD1 axis. Exp Cell Res 2016; 349: 273-281.
 TMR Modern Herbal Medicine2018年3期
TMR Modern Herbal Medicine2018年3期
- TMR Modern Herbal Medicine的其它文章
- Kangfuxin Fluid on the Treatment of Ulcerative Colitis with Retention Enema: a Systematic Review
- Effect of splitting combination of different components of Zhilong Huoxue Tongyu Capsule on vascular remodeling in hypertension
- Pharmacological effects of Paeoniflorin and Albiflorin on IL-3, GM-CSF, IL-6 and TNF-α in the rats of syndrome of stagnation of liver qi and blood deficiency
- Neuroprotective Effect of Bu-Shen-Huo-Xue Extract against High Glucose-induced Apoptosis in PC12 Cells
- Antioxidative and antiapoptotic effects of (+)-clausenamide on acetaminophen-induced nephrotoxicity in mice
- Immunoregulation and anti-tumor effects of Polyporusus Bellatus:a review of recent research
