AUTHOR INSTRUCTIONS
1. Submission Overview
Before you decide to publish with us, please read the following items carefully and make sure that you are well aware of our policies and requirements.
1.1 Topic Suitability
The topic of the manuscript must fit the scope of the journal. Please refer to Aims and Scope for more information.
1.2 Open Access and Copyright
The journal adopts Gold Open Access publishing model and distributes content under the Creative Commons Attribution 4.0 International License. Please make sure that you are well aware of these policies.
1.3 Publication Fees
Authors are required to pay Article Processing Charges of 299 US Dollars after the manuscript is officially accepted. For more details, please refer to Article Processing Charges.
1.4 Language Editing
All submissions are required to be presented clearly and cohesively in good English. Authors whose first language is not English are advised to have their manuscripts checked or edited by a native English speaker before submission to ensure the high quality of expression. A well-organized manuscript in good English would make the peer review even the whole editorial handling more smooth and efficient.
If needed, authors are recommended to consider the language editing services provided by Charlesworth to ensure that the manuscript is written in correct scientific English before submission. Authors who publish with OAE journals enjoy a special discount for the services of Charlesworth.
More details are available at http://www.charlesworthauthorservices.com/.
1.5 Work Funded by the National Institutes of Health
If an accepted manuscript was funded by National Institutes of Health (NIH), the author may inform editors of the NIH funding number. The editors are able to deposit the paper to the NIH Manuscript Submission System on behalf of the author.
2. Submission Preparation
2.1 Cover Letter
A cover letter is required to be submitted accompanying each manuscript. It should be concise and explain why the study is significant, why it fits the scope of the journal, and why it would be attractive to readers, etc.
Here is a guideline of a cover letter for authors’ consideration:
In the first paragraph: include the title and type (e.g., Original Article, Review, Case Report, etc.) of the manuscript, a brief on the background of the study, the question the author sought out to answer and why;
In the second paragraph: concisely explain what was done, the main findings and why they are significant;
In the third paragraph: indicate why the manuscript fits the Aims and Scope of the journal, and why it would be attractive to readers;
In the fourth paragraph: confirm that the manuscript has not been published elsewhere and not under consideration of any other journal. All authors have approved the manuscript and agreed on its submission to the journal. Journal’s specific requirements have been met if any.
If the manuscript is contributed to a special issue, please also mention it in the cover letter.
If the manuscript was presented partly or entirely in a conference, the author should clearly state the background information of the event, including the conference name, time and place in the cover letter.
2.2 Types of Manuscripts
There is no restriction on the length of manuscripts, number of figures, tables and references, provided that the manuscript is concise and comprehensive. The journal publishes Original Article, Review, Meta-analysis, Case Report, Commentary, etc.For more details about paper type, please refer to the following table.

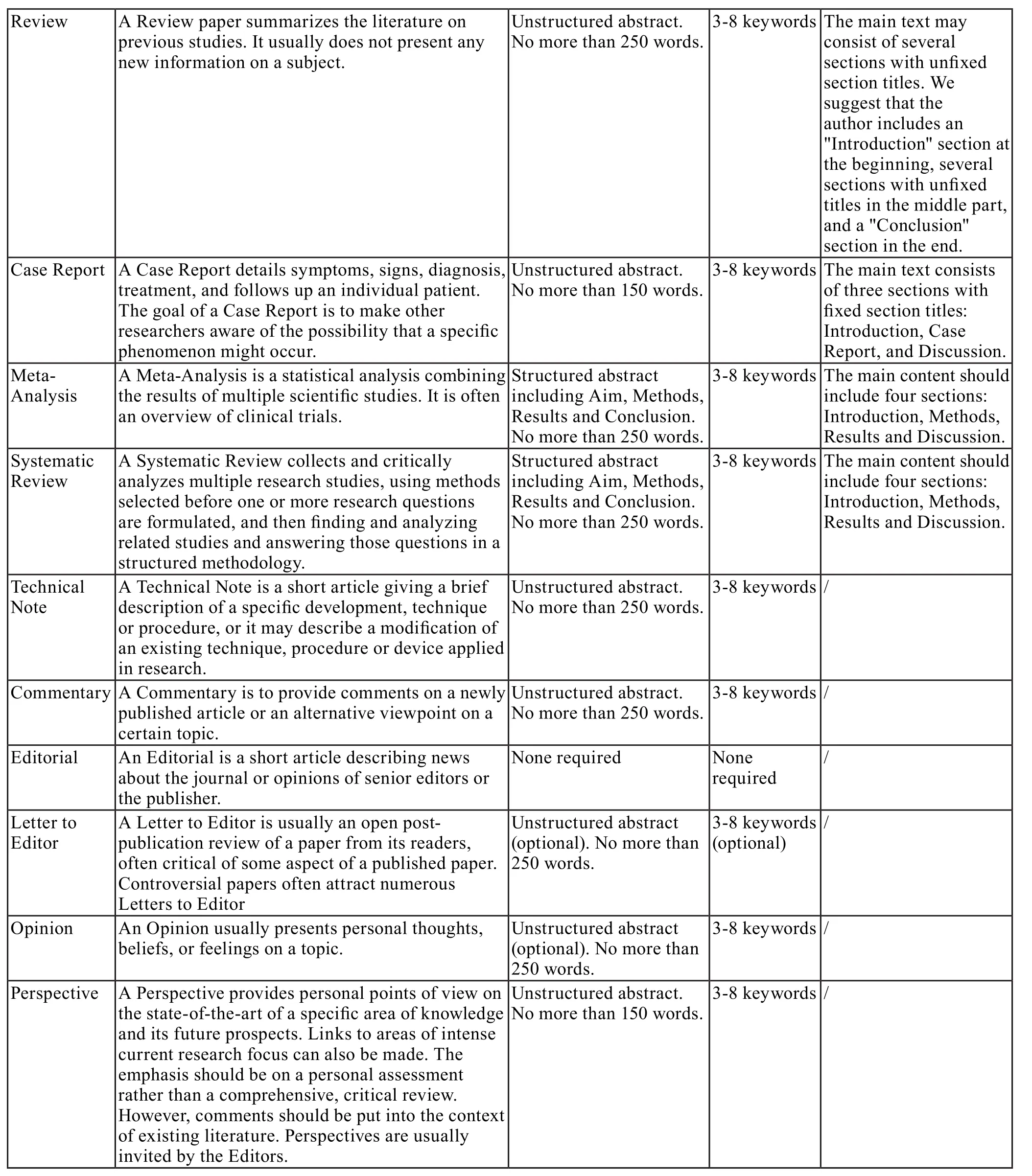
2.3 Manuscript Structure
2.3.1 Front Matter
2.3.1.1 Title
The title of the manuscript should be concise, specific and relevant, with no more than 16 words if possible. When gene or protein names are included, the abbreviated name rather than full name should be used.
2.3.1.2 Authors and Affiliations
Authors’ full names must be listed. The initials of middle names can be provided. Institutional addresses and email addresses for all authors should be listed. At least one author should be designated as corresponding author. In addition,corresponding authors are suggested to provide their Open Researcher and Contributor ID upon submission. Please note that any change to authorship is not allowed after manuscript acceptance.
2.3.1.3 Abstract
The abstract should be a single paragraph with word limitation and specific structure requirements (for more details please refer to Types of Manuscripts). It usually describes the main objective(s) of the study, explains how the study was done,including any model organisms used, without methodological detail, and summarizes the most important results and their significance. The abstract must be an objective representation of the study: it is not allowed to contain results which are not presented and substantiated in the manuscript, or exaggerate the main conclusions. Citations should not be included in the abstract.
2.3.1.4 Keywords
Three to eight keywords should be provided, which are specific to the article, yet reasonably common within the subject discipline.
2.3.2 Main Text
Manuscripts of different types are structured with different sections of content. Please refer to Types of Manuscripts to make sure which sections should be included in the manuscripts.
2.3.2.1 Introduction
The introduction should contain background that puts the manuscript into context, allow readers to understand why the study is important, include a brief review of key literature, and conclude with a brief statement of the overall aim of the work and a comment about whether that aim was achieved. Relevant controversies or disagreements in the field should be introduced as well.
2.3.2.2 Methods
Methods should contain sufficient details to allow others to fully replicate the study. New methods and protocols should be described in detail while well-established methods can be briefly described or appropriately cited. Experimental participants selected, the drugs and chemicals used, the statistical methods taken, and the computer software used should be identified precisely. Statistical terms, abbreviations, and all symbols used should be defined clearly. Protocol documents for clinical trials, observational studies, and other non-laboratory investigations may be uploaded as supplementary materials.
2.3.2.3 Results
This section contains the findings of the study. Results of statistical analysis should also be included either as text or as tables or figures if appropriate. Authors should emphasize and summarize only the most important observations. Data on all primary and secondary outcomes identified in the section Methods should also be provided. Extra or supplementary materials and technical details can be placed in supplementary documents.
2.3.2.4 Discussion
This section should discuss the implications of the findings in context of existing research and highlight limitations of the study. Future research directions may also be mentioned.
2.3.2.5 Conclusion
It should state clearly the main conclusions and include the explanation of their relevance or importance to the field.
2.3.3 Back Matter
2.3.3.1 Acknowledgments
Anyone who contributed towards the article but does not meet the criteria for authorship, including those who provided professional writing services or materials, should be acknowledged. Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgments section. This section is not added if the author does not have anyone to acknowledge.
2.3.3.2 Authors’ Contributions
Each author is expected to have made substantial contributions to the conception or design of the work, or the acquisition,analysis, or interpretation of data, or the creation of new software used in the work, or have drafted the work or substantively revised it.
Please use Surname and Initial of Forename to refer to an author’s contribution. For example: made substantial contributions to conception and design of the study and performed data analysis and interpretation: Salas H, Castaneda WV; performed data acquisition, as well as provided administrative, technical, and material support: Castillo N, Young V.
If an article is single-authored, please include “The author contributed solely to the article.” in this section.
2.3.3.3 Availability of Data and Materials
In order to maintain the integrity, transparency and reproducibility of research records, authors should include this section in their manuscripts, detailing where the data supporting their findings can be found. Data can be deposited into data repositories or published as supplementary information in the journal. Authors who cannot share their data should state that the data will not be shared and explain it. If a manuscript does not involve such issue, please state “Not applicable.” in this section.
2.3.3.4 Financial Support and Sponsorship
All sources of funding for the study reported should be declared. The role of the funding body in the experiment design,collection, analysis and interpretation of data, and writing of the manuscript should be declared. Any relevant grant numbers and the link of funder’s website should be provided if any. If the study is not involved with this issue, state “None.” in this section.
2.3.3.5 Conflicts of Interest
Authors must declare any potential conflicts of interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. If there are no conflicts of interest, please state “All authors declared that there are no conflicts of interest.” in this section. Some authors may be bound by confidentiality agreements.In such cases, in place of itemized disclosures, we will require authors to state “All authors declare that they are bound by confidentiality agreements that prevent them from disclosing their conflicts of interest in this work.”. If authors are unsure whether conflicts of interest exist, please refer to the “Conflicts of Interest” of OAE Editorial Policies for a full explanation.
2.3.3.6 Ethical Approval and Consent to Participate
Research involving human subjects, human material or human data must be performed in accordance with the Declaration of Helsinki and approved by an appropriate ethics committee. An informed consent to participate in the study should also be obtained from participants, or their parents or legal guardians for children under 16. A statement detailing the name of the ethics committee (including the reference number where appropriate) and the informed consent obtained must appear in the manuscripts reporting such research.
Studies involving animals and cell lines must include a statement on ethical approval. More information is available at Editorial Policies.
If the manuscript does not involve such issue, please state “Not applicable.” in this section.
2.3.3.7 Consent for Publication
Manuscripts containing individual details, images or videos, must obtain consent for publication from that person, or in the case of children, their parents or legal guardians. If the person has died, consent for publication must be obtained from the next of kin of the participant. Manuscripts must include a statement that a written informed consent for publication was obtained. Authors do not have to submit such content accompanying the manuscript. However, these documents must be available if requested. If the manuscript does not involve this issue, state “Not applicable.” in this section.
2.3.3.8 Copyright
Authors retain copyright of their works through a Creative Commons Attribution 4.0 International License that clearly states how readers can copy, distribute, and use their attributed research, free of charge. A declaration “? The Author(s)2018.” will be added to each article. Authors are required to sign License to Publish before formal publication.
2.3.3.9 References
References should be numbered in order of appearance at the end of manuscripts. In the text, reference numbers should be placed in square brackets and the corresponding references are cited thereafter. All authors’ names are required to be listed in the references. Abbreviations of the journals should be provided on the basis of Index Medicus. Information from manuscripts accepted but not published should be cited in the text as “Unpublished material” with written permission from the source.
References should be described as follows, depending on the types of works:

Types Examples Journal articles by individual authors Parija SC, Ravinder PT, Shariff M. Detection of hydatid antigen in the fluid samples from hydatid cysts by co-agglutination. Trans R Soc Trop Med Hyg 1996;90:255-6.Organization as author Diabetes Prevention Program Research Group. Hypertension, insulin, and proinsulin in participants with impaired glucose tolerance. Hypertension 2002;40:679-86.Both personal authors and organization as author Vallancien G, Emberton M, Harving N, van Moorselaar RJ; Alf-One Study Group. Sexual dysfunction in 1,274 European men suffering from lower urinary tract symptoms. J Urol 2003;169:2257-61.Journal articles not in English Zhang X, Xiong H, Ji TY, Zhang YH, Wang Y. Case report of anti-N-methyl-D-aspartate receptor encephalitis in child. J Appl Clin Pediatr 2012;27:1903-7. (in Chinese)Books Sherlock S, Dooley J. Diseases of the liver and billiary system. 9th ed. Oxford: Blackwell Sci Pub;1993. p. 258-96.

Book chapters Meltzer PS, Kallioniemi A, Trent JM. Chromosome alterations in human solid tumors. In: Vogelstein B, Kinzler KW, editors. The genetic basis of human cancer. New York: McGraw-Hill; 2002. p. 93-113.Online resource FDA News Release. FDA approval brings first gene therapy to the United States. Available from:https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm. [Last accessed on 30 Oct 2017]Conference proceedings Harnden P, Joffe JK, Jones WG, editors. Germ cell tumours V. Proceedings of the 5th Germ Cell Tumour Conference; 2001 Sep 13-15; Leeds, UK. New York: Springer; 2002.Conference paper Christensen S, Oppacher F. An analysis of Koza's computational effort statistic for genetic programming. In: Foster JA, Lutton E, Miller J, Ryan C, Tettamanzi AG, editors. Genetic programming. EuroGP 2002: Proceedings of the 5th European Conference on Genetic Programming;2002 Apr 3-5; Kinsdale, Ireland. Berlin: Springer; 2002. p. 182-91.Unpublished material Tian D, Araki H, Stahl E, Bergelson J, Kreitman M. Signature of balancing selection in Arabidopsis.Proc Natl Acad Sci U S A. Forthcoming 2002.
For other types of references, please refer to U.S. National Library of Medicine.
The journal also recommends that authors prepare references with a bibliography software package, such as EndNote to avoid typing mistakes and duplicated references.
2.3.3.10 Supplementary Materials
Additional data and information can be uploaded as Supplementary Material to accompany the manuscripts. The supplementary materials will also be available to the referees as part of the peer-review process. Any file format is acceptable, such as data sheet (word, excel, csv, cdx, fasta, pdf or zip files), presentation (powerpoint, pdf or zip files), image(cdx, eps, jpeg, pdf, png or tiff), table (word, excel, csv or pdf), audio (mp3, wav or wma) or video (avi, divx, flv, mov, mp4,mpeg, mpg or wmv). All information should be clearly presented. Supplementary materials should be cited in the main text in numeric order (e.g., Supplementary Figure 1, Supplementary Figure 2, Supplementary Table 1, Supplementary Table 2,etc.). The style of supplementary figures or tables complies with the same requirements on figures or tables in main text.Videos and audios should be prepared in English, and limited to a size of 500 MB or a duration of 3 minutes.
2.4 Manuscript Format
2.4.1 File Format
Manuscript files can be in DOC and DOCX formats and should not be locked or protected.
2.4.2 Length
There are no restrictions on paper length, number of figures, or amount of supporting documents. Authors are encouraged to present and discuss their findings concisely.
2.4.3 Language
Manuscripts must be written in English.
2.4.4 Multimedia Files
The journal supports manuscripts with multimedia files. The requirements are listed as follows:
Videos or audio files are only acceptable in English. The presentation and introduction should be easy to understand. The frames should be clear, and the speech speed should be moderate.
A brief overview of the video or audio files should be given in the manuscript text.
The video or audio files should be limited to a duration of 3 min and a size of up to 500 MB.
Please use professional software to produce high-quality video files, to facilitate acceptance and publication along with the submitted article. Upload the videos in mp4, wmv, or rm format (preferably mp4) and audio files in mp3 or wav format.
2.4.5 Figures
Figures should be cited in numeric order (e.g., Figure 1, Figure 2) and placed after the paragraph where it is first cited;
Figures can be submitted in format of tiff, psd, AI or jpeg, with resolution of 300-600 dpi;
Figure caption is placed under the Figure;
Diagrams with describing words (including, flow chart, coordinate diagram, bar chart, line chart, and scatter diagram, etc.)should be editable in word, excel or powerpoint format. Non-English information should be avoided;
Labels, numbers, letters, arrows, and symbols in figure should be clear, of uniform size, and contrast with the background;Symbols, arrows, numbers, or letters used to identify parts of the illustrations must be identified and explained in the legend;
Internal scale (magnification) should be explained and the staining method in photomicrographs should be identified;
All non-standard abbreviations should be explained in the legend;
Permission for use of copyrighted materials from other sources, including re-published, adapted, modified, or partial figures and images from the internet, must be obtained. It is authors’ responsibility to acquire the licenses, to follow any citation instruction requested by third-party rights holders, and cover any supplementary charges.
2.4.6 Tables
Tables should be cited in numeric order and placed after the paragraph where it is first cited;
The table caption should be placed above the table and labeled sequentially (e.g., Table 1, Table 2);
Tables should be provided in editable form like DOC or DOCX format (picture is not allowed);
Abbreviations and symbols used in table should be explained in footnote;
Explanatory matter should also be placed in footnotes;
Permission for use of copyrighted materials from other sources, including re-published, adapted, modified, or partial tables from the internet, must be obtained. It is authors’ responsibility to acquire the licenses, to follow any citation instruction requested by third-party rights holders, and cover any supplementary charges.
2.4.7 Abbreviations
Abbreviations should be defined upon first appearance in the abstract, main text, and in figure or table captions and used consistently thereafter. Non-standard abbreviations are not allowed unless they appear at least three times in the text.Commonly-used abbreviations, such as DNA, RNA, ATP, etc., can be used directly without definition. Abbreviations in titles and keywords should be avoided, except for the ones which are widely used.
2.4.8 Italics
General italic words like vs., et al., etc., in vivo, in vitro; t test, F test, U test; related coefficient as r, sample number as n,and probability as P; names of genes; names of bacteria and biology species in Latin.
2.4.9 Units
SI Units should be used. Imperial, US customary and other units should be converted to SI units whenever possible. There is a space between the number and the unit (i.e., 23 mL). Hour, minute, second should be written as h, min, s.
2.4.10 Numbers
Numbers appearing at the beginning of sentences should be expressed in English. When there are two or more numbers in a paragraph, they should be expressed as Arabic numerals; when there is only one number in a paragraph, number < 10 should be expressed in English and number > 10 should be expressed as Arabic numerals. 12345678 should be written as 12,345,678.
2.4.11 Equations
Equations should be editable and not appear in a picture format. Authors are advised to use either the Microsoft Equation Editor or the MathType for display and inline equations.
 Journal of Cancer Metastasis and Treatment2018年5期
Journal of Cancer Metastasis and Treatment2018年5期
- Journal of Cancer Metastasis and Treatment的其它文章
- Anti-oxidation properties of leaves, skin, pulp, and seeds extracts from green papaya and their anticancer activities in breast cancer cells
- Management of metastatic esophagogastric junction adenocarcinoma
- Treatment strategy for metastatic gastric cancer in Japan
- Conversion surgery for stage IV gastric cancer
- The combined analysis of solid and liquid biopsies provides additional clinical information to improve patient care
- Hypoxia in prostate cancer
