2017 Cosmetic Regulatory News in AP
Japan released amendment of standard of quasi drug ingredients 2006
Japan MHLW revised its overall standards for raw ingredients used in quasi drug on 30 Mar, 2017 (Table 1 and Table 2). The amendment mainly refers to general testing methods changing of some items and quality standard modification of several ingredients.
This amendments of Standards will be effective on 30 Sep 2018, and before this date, there will a grace period.
Selected Reason: JSQI is the overarching technical standards for quasi drugs, defining the positive lists of ingredients and introducing the testing methods. The regulation is being revised continually.
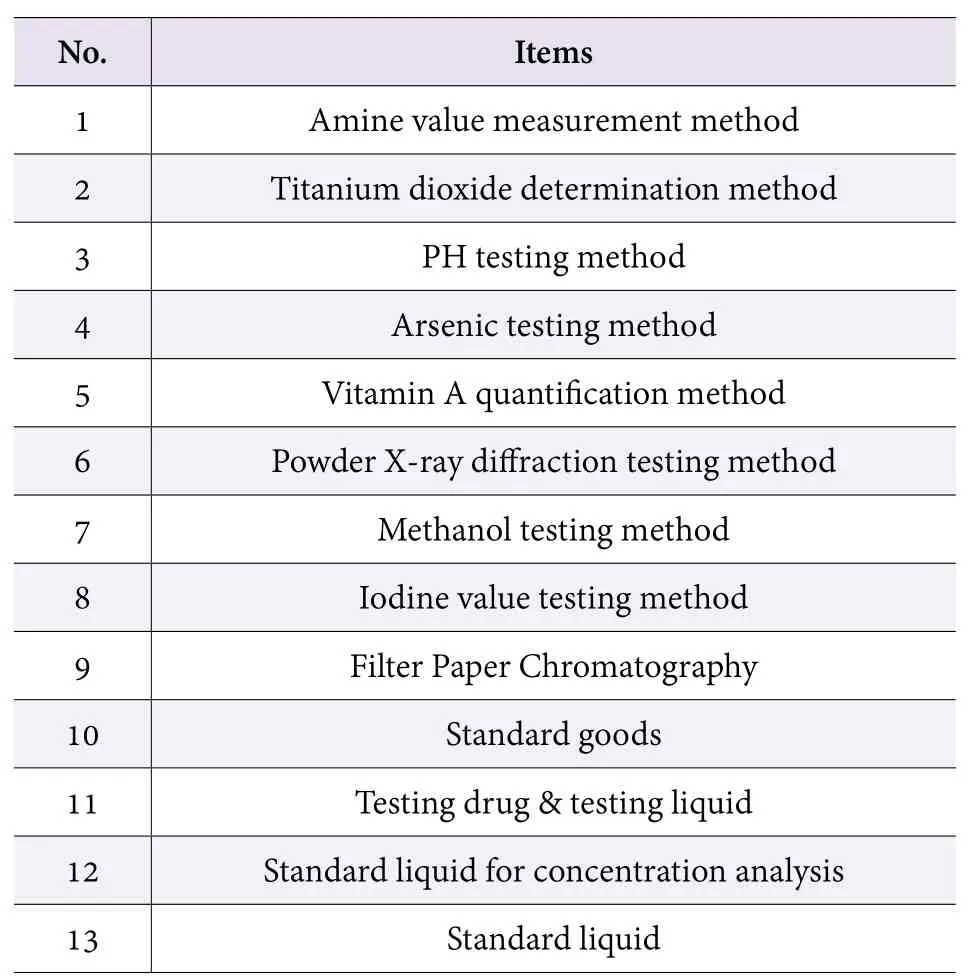
Table 1. Modifications in Part 1 (generally about method)
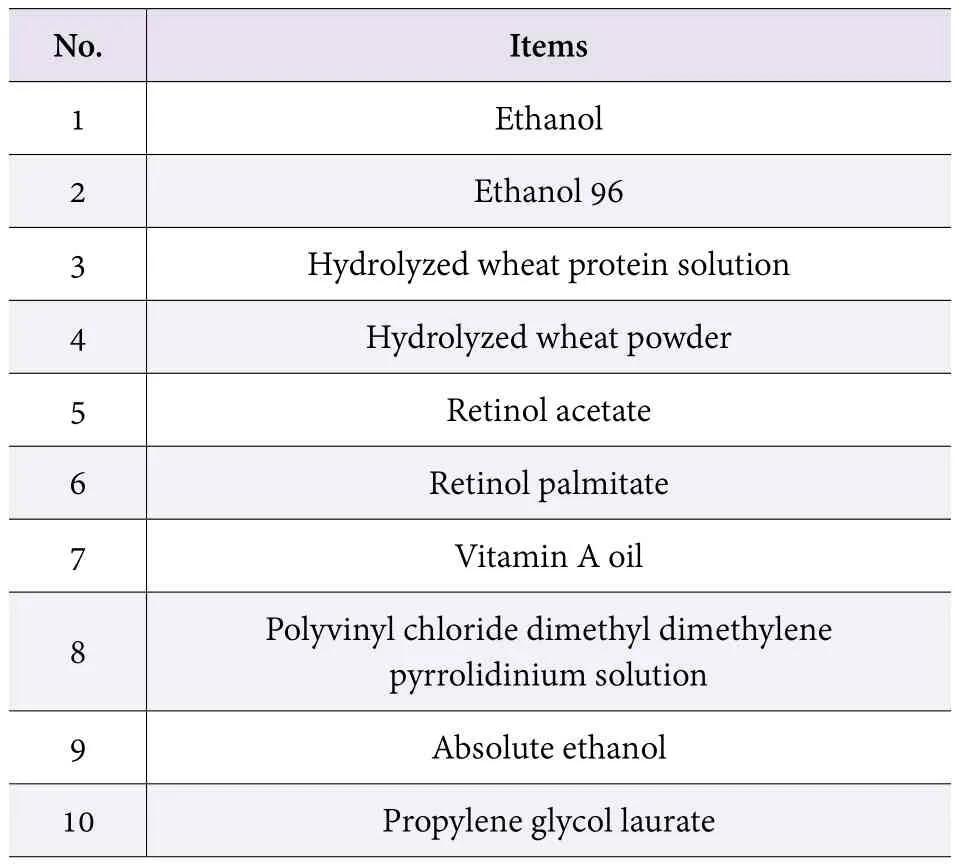
Table 2. Modifications in Part 2 (generally about form and quality change of ingredients)
Skin Cosmetics with Specific Duration of Application Now Require Multiple Dermal Irritation Tests
Skin Cosmetics claiming an exact duration of application (e.g masks, facial scrub) require multiple dermal irritation test. This update contrasts with the previous requirement to conduct a single acute dermal irritation test. Meanwhile, expert panels from the technical review committee also discussed and tentatively agreed on the implementation of a 24h ocular irritation test.
Selected Reason: The changes of test methods haven’t been publicly declared but have been already implemented in all testing institutions in China. It may affect the time and success rate of cosmetic pre-registration test.
ASEAN revised twice the standards of ingredients used in cosmetics
From 2016 to 2017, ASEAN continuingly revised cosmetic ingredients standards and added new ingredients requirements to each standard list. The majority of recent amendments are already in force, and the rest will be implemented on 30 Nov 2017 (Table 3).
Chinese Taiwan FDA revised limits and requirements of TiO2 used in cosmetics
The limit clearly divided the feature of cosmeticsusing TiO2by the concentration of TiO2used. Cosmetics containing TiO2within 25% are designated as general cosmetics and exceeding 25% are regarded as medicated cosmetics and registration is required.
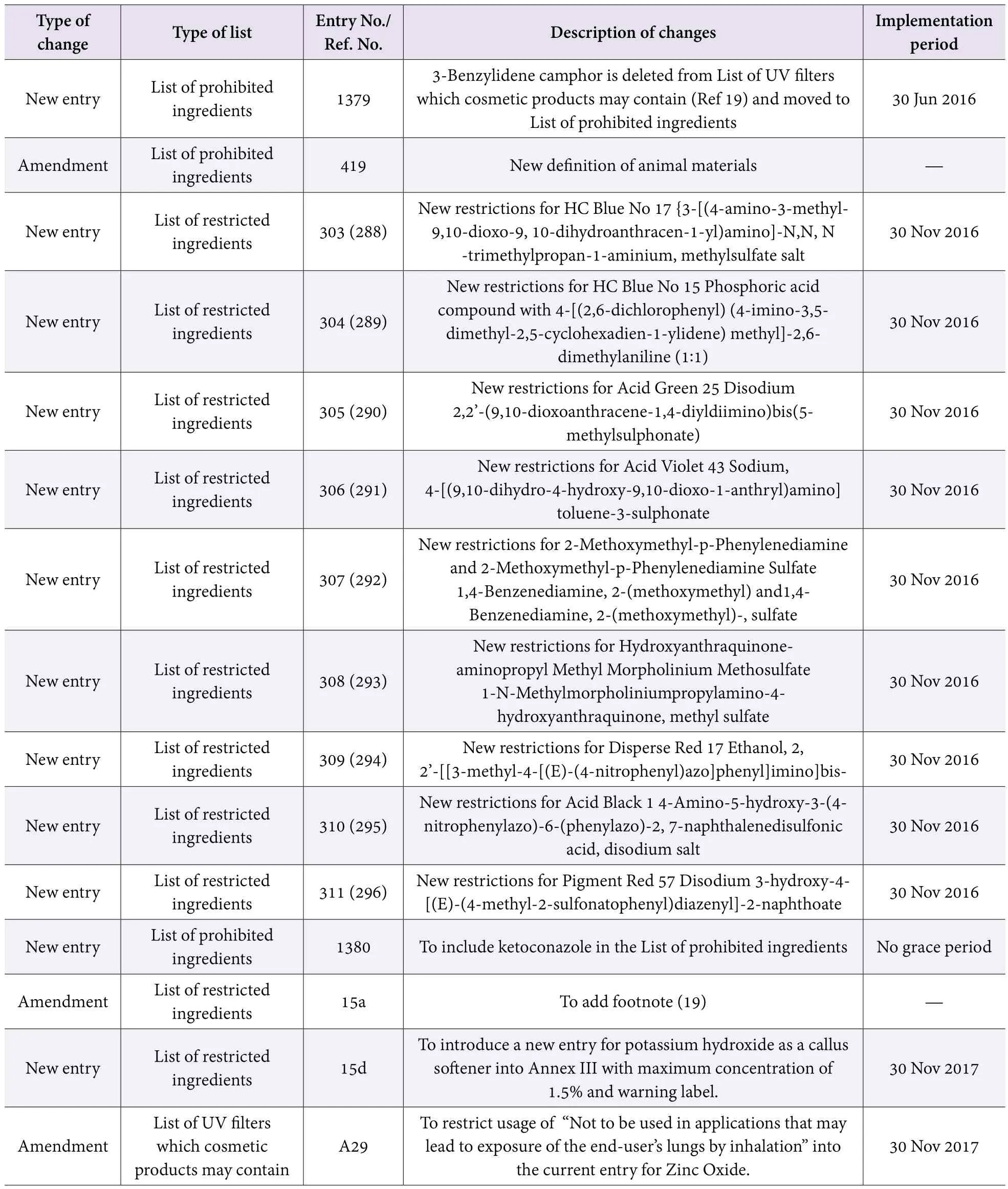
Table 3. Summary of changes to cosmetic ingredient lists of ASEAN
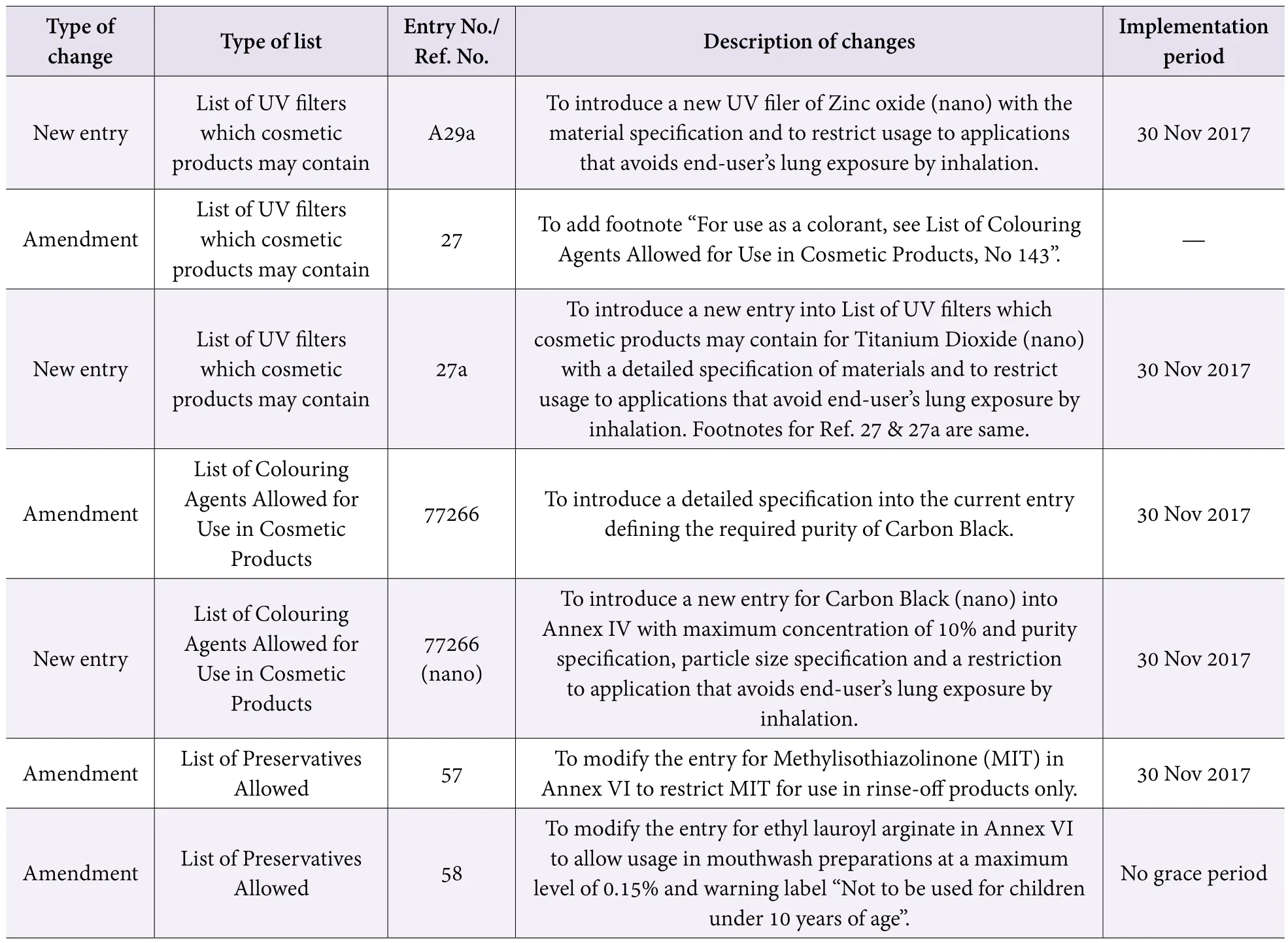
Table 3. Summary of changes to cosmetic ingredient lists of ASEAN (Continued)
Chinese Taiwan FDA amended the provision of controlling titanium dioxide in cosmetics last week.
Cosmetics which contain titanium dioxide (concentration within 25%, exclude Nano sprayers) are designated as general cosmetics, and don’t require registration. In this circumstance, already registered cosmetics don’t have to renew their certificate after expiration. When cosmetics contain both titanium dioxide and Nano titanium dioxide,the total concentration cannot surpass 25%.
Cosmetics which contain titanium dioxide (concentration exceeding 25%) or sprayers contain Nano titanium dioxide are still regarded as medicated cosmetics and registration is required. For registration, a new chemical compound ingredient technical specification should be provided.
When a cosmetic with titanium dioxide is designated as a sunscreen, and the manufacturer has claimed or labeled its SPF, an SPF test report should be prepared by the manufacturing plant or retailers for reference by TFDA.
Selected Reason: TiO2is an ingredient which is heatedly discussed worldwide. TiO2can be used either as whitening or sunscreen functional ingredient, and it is considered as a cancel leading substance so the concentration limits in cosmetics are discussed very often.
China updated 5 cosmetic product industry standards
5 new industry standards (QB) released and implemented on 1 October 2017. These 5 items are mask, depilatory cream,the ingredient iodopropynyl butylcarbamate (CAS: 55406-53-6), the ingredient benzethonium chloride (CAS: 121-54-0) and the ingredient allantoin (CAS: 97-59-6).
Selected Reason: In the new standard, the terminology,definition, classification, requirement, testing methods,inspection principles of the product are revised. It should be minded when manufacturing these cosmetics.
Chinese Taiwan revised medicated cosmetics and colorants registration standard
The standards encompass notable amendments. They allow similar products from same company to share a single certificate; allow secondary manufacturer to register(which is uncovered in dossiers required), and allow medical and poisonous ingredients to follow the standards of EU, US and Japan.
On 22 Feb, 2017, Chinese Taiwan FDA officially issued the revised version of Standards for Examination and Registration of Medicated Cosmetics and Colorants replacing Instructions for Examination and Registration of Medicated Cosmetics and Colorants.
Selected Reason: In Chinese Taiwan, medicated cosmetics need registration before entering the market. The standard specified the requirements when applying for the registration certificate for medicated cosmetics. The revision should be noted if one wants to export medicated cosmetics to Taiwan.
Australia replaced the original NICNAS chemical management scheme by AICS
Australia will reform its National Industry Chemicals Notification and Assessment Scheme, the statutory scheme to regulate chemicals including cosmetics. The major changes affecting the cosmetics industry will be fully implemented by 1 Sep. 2018; however, some of the proposed changes are already effective since 1 Sep. 2016.
To reduce regulatory burden and help Australia becomes more competitive, the reform involves:
1. rebalancing pre-and post-market regulatory requirements to match the risk profile of a new chemical;
2. streamlining the existing risk assessment process for new and existing chemicals;
3. greater utilization of international assessment criteria;
4. more appropriate compliance tools.
Officials predict that the changes will lower the costs of market entry and a large number of chemicals should be subject to significantly reduced costs. It is expected that the overall reduction in regulatory burden to industry per year will be $23 million;
For new chemicals, pre- and post-market regulatory controls will be rebalanced by applying a risk based framework to match assessment standards to the risk profile of individual chemicals (Table 4).
Selected Reason: In Australia, cosmetics are classified as chemicals and managed under the chemical regulatory scheme. The total reform of the scheme does affect the manufacturing and importing of cosmetics. The new scheme introduced a risk assessment and chemicals are divided into 5 categories based on estimated hazardous risk.
Korea expanded functional cosmetic scope
These categories: 1) hair dye, decolorant, bleach agent;2) epilating agent; 3) anti-hair loss agent; 4) preparation of relieving acne in bath solvents are designated as functionalcosmetics from 30 May 2017. Several supplementary regulations and requirements are simultaneously amended(Table 5).
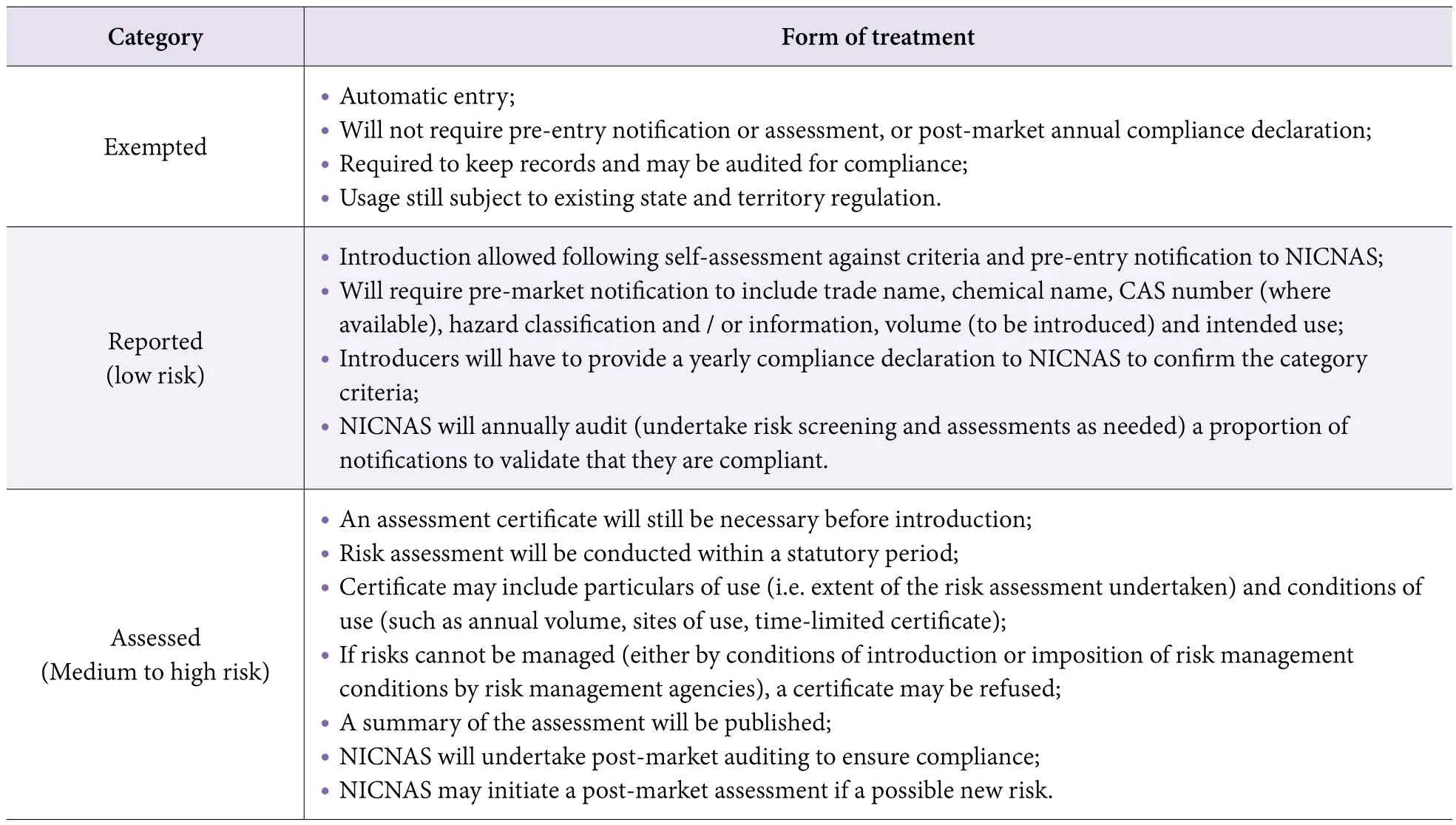
Table 4. Revised contexts

Table 5. Latest regulation updates in Korea
Korea is among the most lucrative cosmetic market which attracts quantity of cosmetic products importing in. Korea Ministry of Food and Drug Safety (MFDS)regulates cosmetics by issueing basic “Cosmetic Act”.To enter Korea market, cosmetics enterprises have to undergo several registration process. To see general cosmetic regulation compliance in Korea, please visit:Cosmepedia: Korea Cosmetic Regulation
Selected Reason: Expanding Functional Cosmetic Scope might be the most significant change for the first half year of 2017 in Korea cosmetic industry.Implemented on 30 May, 2017, the new categorization system designated 4 quasi-drugs as functional cosmetics.
China Cross-border Ecommerce (CBEC)policy
The management of CBEC in China is still in an unclear stage. Early this spring, the Ministry of Commerce of China spoke to regard CBEC products imported to China as personal articles. But on September the Ministry announced to extend the transition period till the end of 2018. Therefore the progress of managing CBEC products seemed suspended.
Selected Reason: CBEC used to be a friendly method for importing foreign products to China because it doesn’t require complex compliance procedures. But in 2016,there released a series of policies to tighten up the control of the new importing portal. But the tendency seemingly drew back in 2017. Therefore the progress of managing CBEC products seemed suspended.
Pudong pilot spot for imported non special use cosmetics filing management
Early in 2017, CFDA released the announcement to set pudong pilot spot as filing practice for non-special imported cosmetics. This is a constructive attempt to simplify cosmetic compliance process in China, and multiple of foreign enterprises get involved in the practice this year. In May 2016,the State Council of China announced that it was to adjust registration management to filing management for nonspecial use cosmetics imported for the first time in Shanghai Pudong New Area. To implement the notice, China Food and Drug Administration which is responsible for the formulation of cosmetic regulations and registration / filing of cosmetics released further enforcement rules on 17 Jan 2017.
As stipulated in the announcement, from 1 Mar, 2017 to 21 Dec, 2018 if a non-special use cosmetic product is imported through the port of Shanghai Pudong New Area for the first time and the responsible agent is located in the New Area, the product will be subject to filing management instead of registration. The manufacturer or domestic responsible agent shall apply for filing prior to import. An online filing system will be established for the applications.Only after obtaining a filing certificate on the online system will the product be permitted to be imported and distributed.
For products filed in accordance with the announcement,if they are imported though other ports, the manufacturer or domestic responsible agent shall cancel the filing, then register their products according to the normal requirements for first import non-special use cosmetics.
CFDA and CIQ in China will together implement the rules. CFDA will be in charge of the filing while CIQ will be responsible for checking the filing certification.
Selected Reason: The administrative simplification in China is a huge trend relating to all the industries. Among them, cosmetic importation registration has long been suggested to subject to a simpler process. Pudong trial is the first step and the practice affected crucial stakeholders in this field.
 China Detergent & Cosmetics2018年2期
China Detergent & Cosmetics2018年2期
- China Detergent & Cosmetics的其它文章
- Sensory Evaluation of Hydroxy Ethyl Cellulose (HEC) in Facial Mask
- Application of “Youthful Curve of Ocular Region” in the Research of Asians Aging
- Latest Update of Cosmetic Regulations in China Since 2017
- How to Comply with the Pilot Policy of China for the Import of Non-special Use Cosmetics
- Analysis of Regulatory Policies and Registration / Record Keeping Status of Domestic Cosmetics in China
- Middle Class pivotal to China’s Beauty Market
