Ultrasound features of extranodal extension in the metastatic cervical lymph nodes of papillary thyroid cancer: a case-control study
Jiali Mu, Xiaofeng Liang, Fangxuan Li, Juntian Liu, Sheng Zhang, Jing Tian
1Department of Ultrasound; 2Department of Cancer Prevention Center, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin’s Clinical Research Center for Cancer, Tianjin 300060, China; 3Department of Ultrasound, The Secondary Hospital of Tianjin Medical University, Tianjin 300211, China
Introduction
Papillary thyroid carcinoma is the most common type of thyroid cancer, representing 75% to 85% of all thyroid cancer cases1. Papillary thyroid carcinoma has a low mortality rate,and the overall 5-year and 10-year survival rates are 96% to 97% and 93%, respectively2. Well-established prognostic factors for thyroid carcinomas include age of 55 years or older, tumor size, extracapsular invasion, lymph nodal spread, and distant metastasis3. Lymph nodal spread, as suggested by the TNM staging system from the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) is classified as: N0, no node metastasis; N1a, metastasis to level VI or VII; and N1b,metastasis to level I, II, III, IV or V retropharyngeal lymph nodes4. However, during malignant tumor invasion, cancer cells not only spread to areas surrounding regional lymph nodes along lymphatic vessel, but also can invade the lymph node capsule and beyond, which is considered extracapsular spreading or extranodal extension5,6. Extranodal extension is histologically identified as an extension of the tumor through the lymph node capsule, into the adjacent perinodal soft tissue, and contributes to severity of lymph node involvement. Despite its clinical importance, extranodal extension has been left out of the TNM classification system.
Several studies have demonstrated that extranodal extension is an important risk factor for the progression and poor prognosis of papillary thyroid cancer. In the study of Ito et al.7, extranodal extension was found to be associated with increased nodal persistence after initial resection and reduced biochemical response to radioiodine treatment, and extranodal extension is an independent marker of poor survival. Lango et al.8reported that extranodal extension is associated with distant metastasis and independently predicted the eventual progression of extranodal extension to systemic disease. Yamashita et al.9and Ito et al.10both showed that extranodal extension is associated with worse disease free survival and cause-specific survival. The study of Wu et al.11found that extranodal extension is a stronger predictor of survival than the location of metastatic lymph nodes (for example, N1a vs. N1b). Furthermore, similar survival was observed between patients with IVa or IVb-stage thyroid cancer without extranodal extension and patients with stage III thyroid cancer with extranodal extension11.Given the prognostic value of extranodal extension in predicting survival, and the association with disease progression, the ability to diagnose extranodal extension before surgery is desirable and will guide the extent of the central neck dissection. However, currently, the presence of extranodal extension only can be diagnosed during or after surgery.
Ultrasonography is a reliable method for diagnosing thyroid carcinoma and has had high accuracies in the diagnosis of malignant thyroid nodal12, and assessment of cervical lymph node13, metastases. Currently, ultrasonography is routinely used in preoperative settings to evaluate metastatic nodal diseases from thyroid malignancies. Wellestablished and specific ultrasound features correlate with the presence of metastatic lymph nodes, such as the presence of enlarged lymph nodes with aspect ratios < 2, cystic areas or microcalcifications within the node, and hypervascularity14,15.
To our knowledge, whether ultrasound can preoperatively determine the presence of extranodal extension remains unknown. Qualliotine et al.16reported an association between ultrasound characteristics and extranodal extension in metastatic papillary thyroid carcinoma, via a retrospective analysis. Specifically, Qualliotine et al.16reported that node matting and nodes with cystic contents greater than 50%were significantly associated with extranodal extension, and their study has been the only report to evaluate specific sonographic criteria for extranodal extension in patients with metastatic papillary thyroid carcinoma. However, due to the limited cases, only 11 patients were enrolled into their study,and a larger patient population will be required to obtain more reliable conclusions. In the present study, we analyzed clinicopathologic and ultrasound features of 60 patients with papillary thyroid cancer with extranodal extension, to explore important preoperative ultrasound features that are associated with pathologic extranodal extension. The specific aim of this study was to identify common and characteristic preoperative ultrasound features that are associated with the pathologic extranodal extension of metastatic papillary thyroid carcinoma.
Patients and methods
Patients
The patient enrollment criteria were: (1) patients who had received ultrasonography and color Doppler flow imaging(CDFI) of the thyroid gland and the whole cervical region,because of suspected thyroid carcinoma, from March 2011 to March 2016 at the Tianjin Medical University Cancer Institute and Hospital; (2) patients who had received thyroidectomies and cervical lymph node dissections from March 2011 to March 2016 at the Tianjin Medical University Cancer Institute and Hospital; (3) patients who had a pathological diagnosis of papillary thyroid carcinoma with lymph node metastasis; and (4) patients who had a pathological diagnosis of extranodal extension-positive metastatic cervical lymph nodes (representative pathological images are shown in Figure 1).
The elimination criteria were: (1) patients who had not received preoperative cervical ultrasound imaging at our institute; (2) patients who had received thyroidectomies at another hospital and only underwent cervical lymph node dissection at our institute; and (3) patients who had incomplete pathophysiologic information. Altogether, 60 patients were enrolled into our study and comprised the observation group.
A 1:2 ratio of matched case to controls was applied, to avoid bias from of clinical feature disparities. A total of 120 patients with papillary thyroid carcinomas with cervical lymph nodes metastases and without extranodal extension comprised the control group (Table 1).

Figure 1 Histologic image of extranodal extension in cervical lymph nodes of papillary thyroid carcinoma patients (H&E staining, 200 ×). (A) A metastatic lymph node at level VI. (B)Extranodal extension of this metastatic lymph node.
This study was approved by the Ethics Committee of Tianjin Cancer Institute and Hospital. Written consent was obtained from each patient.
Ultrasonography examination
The ultrasound examination was performed with a Philips iU22 digital ultrasound scanner system (Philips Bothell,Washington, USA), which was equipped with a liner electronic probe, with a central frequency of 7–12.5 MHz.Patients were maintained in the supine position with the neck slightly extended, to fully expose the anterior region of the neck. All ultrasound images were evaluated and recorded by two independent sonographers, each of whom was blinded to the findings of the other sonographer and the pathology results. If the diagnoses were discordant, a third sonographer mediated to achieve consensus.First, the thyroid gland was scanned, to assess the primary thyroid cancer site. Then, we performed B-mode and color Doppler ultrasound in the whole cervical region to obtain representative images of nodal stations, as defined by Som et al.17. The probe was applied to the neck and moved in the transverse and vertical sections. The three dimensional size(tall, long and wide), aspect ratio (in the tangent plane at maximum diameter, defined as long/tall), location (cervical lymph node level), hilar effacement, margin (well-/illdefined), shape (irregular/regular), node matting (nodes were confluent and matted to each other), cystic area,microcalcifications (defined as calcifications < 1 mm), and central and peripheral blood flow (via CDFI) of the nodes were examined.
Statistical analysis
Statistical analyses were performed using the SPSS software package (version 19.0; SPSS, Chicago, IL, USA). Differenceswith two-tailed t-test P values of less than 0.05 were considered statistically significant. Continuous variables were described by mean ± standard deviation. The independent sample t test was used to compare continuous variables, and the chi-squared and fisher exact tests were applied for the univariate analysis of categorical variables. A logistic regression was applied to the multivariate analysis.
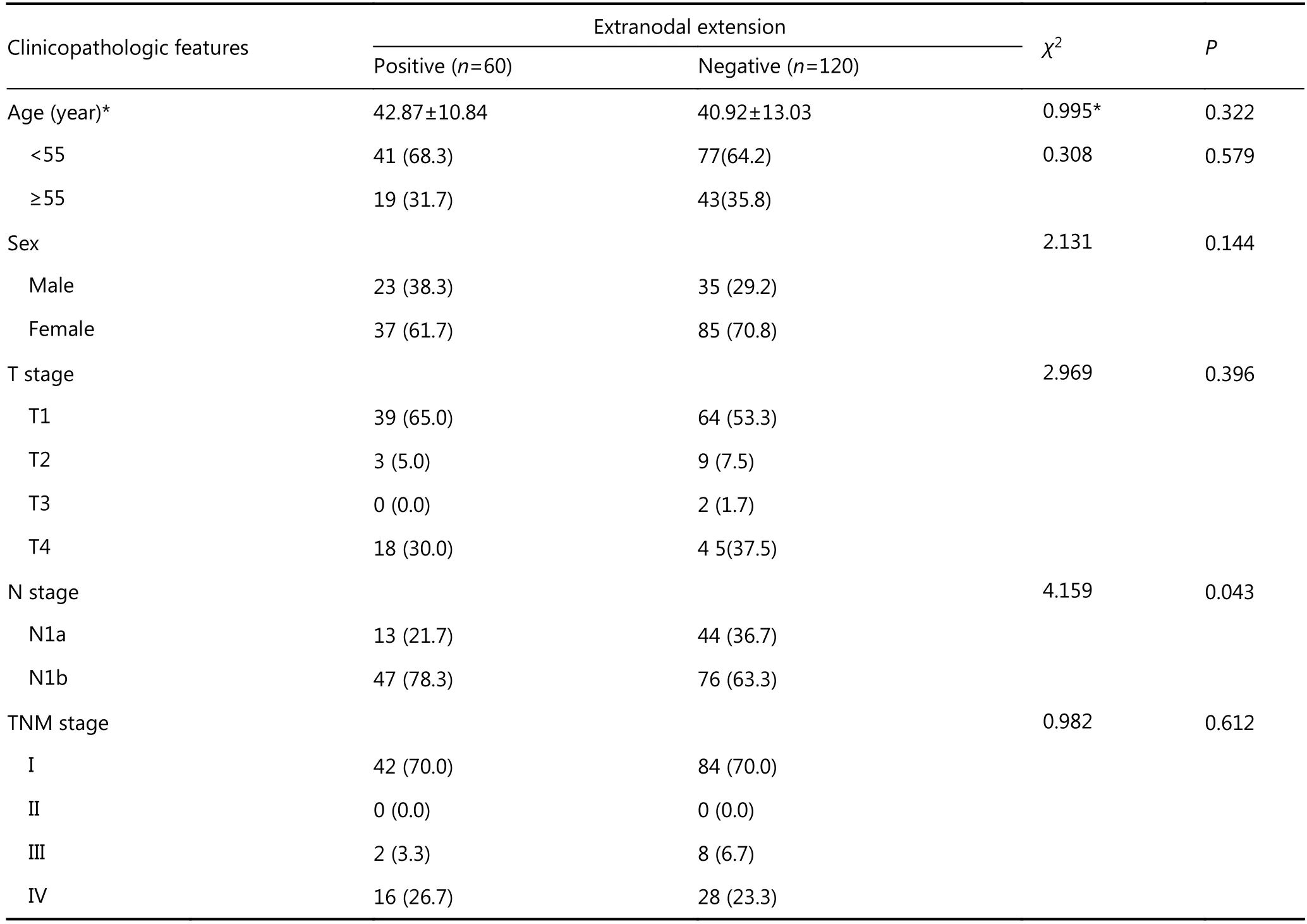
Table 1 Baseline clinicopathologic features of papillary thyroid carcinoma patients with/without extranodal extension
Results
The clinicopathologic features of papillary thyroid carcinomas with extranodal extensionpositive cervical lymph nodes
As shown in Table 1, there was no significant difference in age, age distribution, sex, T stage, TNM stage (all P > 0.05)between patients with papillary thyroid carcinomas who were positive or negative for extranodal extension. However, N1b stage papillary thyroid carcinomas were more frequently found in patients with extranodal extension, compared to those without extranodal extension (78.3% vs. 63.3%, P =0.043).
The extranodal extension patterns in the cervical lymph nodes of patients with papillary thyroid carcinoma
In all 60 patients of the observation group, the greatest frequency of extranodal extension positivity was detected at level VI, followed by levels III, IV, and II. The pattern of extranodal extension positivity is shown in Figure 2.
The ultrasound features of extranodal extension in the cervical lymph nodes of patients with papillary thyroid carcinoma

Figure 2 The pattern of extranodal extension in cervical lymph nodes of papillary thyroid carcinoma patients.

Figure 3 Representative sonogram of extranodal extension positive. (A) Representative sonogram of node matting (white arrows) in cervical lymph nodes with extranodal extension. (B)Representative sonogram of microcalcification (white arrows) in cervical lymph nodes with extranodal extension. (C)Representative sonogram of cystic area (white arrows) in cervical lymph nodes with extranodal extension positive. (D)Representative sonogram of aspect ratio < 2 and larger diameter in cervical lymph nodes with extranodal extension positive.
In our univariate analysis of patients with papillary thyroid carcinoma, extranodal extension-positive lymph nodes showed higher incidences of node matting (Figure 3A),microcalcification (Figure 3B), cystic area (Figure 3C),aspect ratios < 2, and larger diameter (Figure 3D) than extranodal extension-negative lymph nodes (shown in Table 2,P < 0.05). Representative images from these comparisons are shown in Figure 3A-D. All of these significant features were used in our logistic regression multivariate analysis, which demonstrated that the presence of both node matting and cystic area were independent risk factors for extranodal extension [node matting, odds ratio (OR): 4.751, 95%confidence interval (CI): 1.212–18.626, P=0.025; cystic area OR: 2.707, 95% CI: 1.127–6.502, P=0.026], shown in Table 3.
Discussion
Cervical lymph node metastasis is a risk factor for local tumor recurrence in patients with papillary thyroid carcinoma. Previous reports have provided compelling evidence regarding the importance of the metastatic node size, number of positive nodes, and presence of extranodal extension, for the prognosis of patients with papillary thyroid cancer18. Although extranodal extension is not officially recognized in AJCC and UICC staging systems, it has been shown, in a number of studies, to have negative prognosticimpacts. Lango et al.8identified several outcome measures that are associated with extranodal extension in patients with papillary thyroid cancer. These associated outcomes included the diminished probability of a complete biochemical response, the increased probability of structural tumor persistence, and systemic disease progression. Wu et al.11observed that extranodal extension was significantly associated with older age, advanced disease stage, lymph node status N1b (95%), and larger lymph nodes > 1 cm(80%)11. In the present study, N1b stage was more frequently(78.3%) found in extranodal extension-positive papillary thyroid carcinomas than in those that were extranodal extension-negative. Therefore, with respect to our results, the presence of pathological extranodal extension reflects asubset of papillary thyroid carcinomas with biologically aggressive behavior. The clinical significance of the presence of extranodal extension, for disease staging and prognosis,have also been reported for other malignancies, such as head and neck squamous cell carcinoma19, breast cancer6,colorectal cancer20, and gastric cancer21.

Table 2 The ultrasound features of cervical lymph node of papillary thyroid carcinoma patients with/without extranodal extension

Table 3 Multivariate logistic regression for ultrasound features of cervical lymph node correlated to extranodal extension in papillary thyroid carcinoma patients
In coincidence with the lymphatic spread pattern of papillary thyroid carcinoma, which showed lymph nodes metastasis most frequently in level VI, followed by levels III,IV and II22, we found that extranodal extension follows the same pattern. According to the literature, and given the prognostic implications of extranodal extension, the level VI subset would require meticulous and thorough removal of lymphadenopathy8,18. The determination of extranodal extension through preoperative ultrasound may represent clinical disease features and improve preoperative risk stratifications, through delineating the need for additional surgical expertise and central neck dissection evaluation.Preoperative ultrasound, for the detection of lymph node metastases in the central and lateral compartments, has been the standard of care for patients with papillary thyroid cancer. In general, a larger size, irregular shape, unclear margin, eccentric cortical thickening, cystic changes, cortical hyperechogenicity, microcalcification, and hypervascularity were predictable findings for lymph node metastasis14,15.Until now, data from preoperative ultrasound evaluations for the diagnoses of extranodal extension in papillary thyroid cancer have rarely been available.
In 2010, Misselt et al.22studied the ultrasound characteristics of extranodal extension in breast cancer, and found that the features that were significantly associated with extranodal extension included node matting, unclear margins, and hilar replacement, on univariable analysis, and both node matting and unclear margins were independently associated on multivariable analysis. Until now, only Qualliotine et al.16had discussed the ability of ultrasound for evaluating extranodal extension in papillary thyroid cancer.This investigation was a retrospective pilot study, and based on 11 patients with extranodal extension, they proposed that extranodal extension may be another clinically significant characteristic of nodal metastases. In their study, the following ultrasound characteristics were significantly associated with extranodal extension positivity: node matting, perinodal edema, unclear margins, and cystic areas.
In the present study, we demonstrated that larger tumor size, aspect ratio, microcalcification, node matting, and cystic areas were associated with the determination of extranodal extension, through our univariable analysis, while node matting and cystic areas independently associated with extranodal extension in our multivariable analysis. Larger tumor size, especially tumors with aspect ratios < 2, is a significant risk factor for extranodal extension, and this conveys the fact that malignant cells grow across normal tissue planes because of the invasiveness of cancer cells. In addition, node matting is another indicator of typical tumor invasiveness. The presentation of microcalcification is caused by rapid cell growth and insufficient blood supply to the metastatic site, which cause local fibrous tissue hyperplasia and calcium deposition, while cystic areas are also associated with rapid cell growth and insufficient blood supply, which cause putrescence and liquescence in the focus14,15. All these features are associated with the biologically aggressive behavior of cancer cells.
Our study provides more clinical evidence for future evaluations of extranodal extension, via preoperative ultrasound, in patients with papillary thyroid cancer. Future studies should aim to provide useful indices for extranodal extension evaluations and preoperative risks stratifications.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
1.Al-Brahim N, Asa SL. Papillary thyroid carcinoma: an overview.Arch Pathol Lab Med. 2006; 130: 1057-62.
2.Ito Y, Kihara M, Takamura Y, Kobayashi K, Miya A, Hirokawa M,et al. Prognosis and prognostic factors of papillary thyroid carcinoma in patients under 20 years. Endocr J. 2012; 59: 539-45.
3.Lamartina L, Grani G, Arvat E, Nervo A, Zatelli MC, Rossi R, et al.8th edition of the AJCC/TNM staging system of thyroid cancer:what to expect. Endocr Relat Cancer. 2017; 25: L7-11.
4.Suh S, Kim YH, Goh TS, Lee J, Jeong DC, Oh SO, et al. Outcome prediction with the revised American joint committee on cancer staging system and American thyroid association guidelines for thyroid cancer. Endocrine. 2017; 58: 495-502.
5.Moritani S. Impact of invasive extranodal extension on the prognosis of patients with papillary thyroid carcinoma. Thyroid.2014; 24: 1779-83.
6.Palamba HW, Rombouts MC, Ruers TJM, Klinkenbijl JHG,Wobbes T. Extranodal extension of axillary metastasis of invasive breast carcinoma as a possible predictor for the total number of positive lymph nodes. Eur J Surg Oncol. 2001; 27: 719-22.
7.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A.Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012; 36: 1274-8.
8.Lango M, Flieder D, Arrangoiz R, Veloski C, Yu JQ, Li TY, et al.Extranodal extension of metastatic papillary thyroid carcinoma:correlation with biochemical endpoints, nodal persistence, and systemic disease progression. Thyroid. 2013; 23: 1099-105.
9.Yamashita H, Noguchi S, Murakami N, Toda M, Yamashita H,Uchino S, et al. Extracapsular invasion of lymph node metastasis. A good indicator of disease recurrence and poor prognosis in patients with thyroid microcarcinoma. Cancer. 1999; 86: 842-9.
10.Ito Y, Hirokawa M, Jikuzono T, Higashiyama T, Takamura Y, Miya A, et al. Extranodal tumor extension to adjacent organs predicts a worse cause-specific survival in patients with papillary thyroid carcinoma. World J Surg. 2007; 31: 1196-203.
11.Wu MH, Shen WT, Gosnell J, Duh QY. Prognostic significance of extranodal extension of regional lymph node metastasis in papillary thyroid cancer. Head Neck. 2015; 37: 1336-43.
12.Wei X, Li Y, Zhang S, Gao M. Thyroid imaging reporting and data system (TI-RADS) in the diagnostic value of thyroid nodules: a systematic review. Tumour Biol. 2014; 35: 6769-76.
13.Khanna R, Sharma AD, Khanna S, Kumar M, Shukla RC.Usefulness of ultrasonography for the evaluation of cervical lymphadenopathy. World J Surg Oncol. 2011; 9: 29
14.Sohn YM, Kwak JY, Kim EK, Moon HJ, Kim SJ, Kim MJ.Diagnostic approach for evaluation of lymph node metastasis from thyroid cancer using ultrasound and fine-needle aspiration biopsy.AJR Am J Roentgenol. 2010; 194: 38-43.
15.Yoo YH, Kim JA, Son EJ, Youk JH, Kwak JY, Kim EK, et al.Sonographic findings predictive of central lymph node metastasis in patients with papillary thyroid carcinoma: influence of associated chronic lymphocytic thyroiditis on the diagnostic performance of sonography. J Ultrasound Med. 2013; 32: 2145-51.
16.Qualliotine JR, Coquia SF, Hamper UM, Fakhry C. Association of ultrasound characteristics with extranodal extension in metastatic papillary thyroid carcinoma. JAMA Otolaryngol Head Neck Surg.2016; 142: 263-9.
17.Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000; 174: 837-44.
18.Randolph GW, Duh QY, Heller KS, Livolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012; 22: 1144-52.
19.Lee JR, Choi YJ, Roh JL, Kim JS, Lee JH, Cho KJ, et al. Preoperative contrast-enhanced CT versus 18F-FDG PET/CT evaluation and the prognostic value of extranodal extension for surgical patients with head and neck squamous cell carcinoma. Ann Surg Oncol. 2015;22: 1020-7.
20.Luchini C, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, et al.Significance of the prognostic stratification of extranodal extension in colorectal cancer. Ann Oncol. 2016; 27: 1647
21.Veronese N, Fassan M, Wood LD, Stubbs B, Solmi M, Capelli P,et al. Extranodal extension of nodal metastases is a poor prognostic indicator in gastric cancer: a systematic review and meta-analysis. J Gastrointest Surg. 2016; 20: 1692-8.
22.Misselt PN, Glazebrook KN, Reynolds C, Degnim AC, Morton MJ.Predictive value of sonographic features of extranodal extension in axillary lymph nodes. J Ultrasound Med. 2010; 29: 1705-9.
 Cancer Biology & Medicine2018年2期
Cancer Biology & Medicine2018年2期
- Cancer Biology & Medicine的其它文章
- Ovarian cancer presenting with hypercalcemia: two cases with similar manifestations but different mechanisms
- Primary resistance to crizotinib treatment in a non-small cell lung cancer patient with an EML4-ALK rearrangement: a case report
- Diagnostic value of whole-body MRI with diffusion-weighted sequence for detection of peritoneal metastases in colorectal malignancy
- Clinical significance of miRNA - 106a in non-small cell lung cancer patients who received cisplatin combined with gemcitabine chemotherapy
- Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-β-induced epithelial–mesenchymal transition
- Progress in non-invasive detection of liver fibrosis
