Enhancement of Visible-light Photocatalytic Performance Via TiO2 Nanotube Arrays Loaded with Polypyrrole-polyaniline Copolymer
, , ,
(Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, College of Environmental and Chemical Engineering, Nanchang Hangkong University, Nanchang 330063, China)
1 Introduction
Because long-term drinking of nitrophenol- contaminated water is harmful to human health, it is important to remove nitrophenols from water[1-2]. Up to now, several methods were utilized to remove nitrophenols from water, such as biodegradation, adsorption, electrochemical oxidation, and heterogeneous photocatalytic oxidation[3]. Among these methods, heterogeneous photocatalytic oxidation is regarded as one of the most promising techniques for wastewater treatment[4-5].
Titanium dioxide (TiO2) has captured considerable attention due to its high physico- chemical stability, non-toxicity, low cost, and high photocatalytic activity[6-10]. However, the photocatalytic activity only works under UV light irradiation due to its wide band gap (Eg=3.0-3.2eV), the solar energy conversion efficiency is very low, and the recombination rate of photogenerated electrons and holes is fast, which limit its practical applications[11-12]. Dye sensitization is an effective method to raise the visible-light photocatalytic activity of TiO2, but the application of dye-sensitized TiO2is still restricted due to dye degradation during the photocatalytic process[13-14].
Up to now, conductive polymers with extended π-conjugated electron systems have drawn extensive attention due to their high mobility of charge carriers, high absorption coefficients, high conductivity, effective electron donors and good electron transporters[15-19]. Polypyrrole (PPy) and polyaniline (PANI) are popular conductive polymers due to their high conductivity and excellent environmental stability, and has been used as stable photosensitizers to enhance their solar energy transfer, electronic conductivity and photocatalytic activities of TiO2[20-21].
Conductive polymer composites exhibit catalytic and sensing properties better than those of neat conductive polymers. Our previous study indicated that conductive polypyrrole-polyaniline/TiO2nanoparticles (PPy-PANI/TiO2) showed higher photocatalytic activity than that of polypyrrole/TiO2(PPy/TiO2) and polyaniline/TiO2(PAIN/TiO2) under visible-light illumination.
The powder-form photocatalysts are difficult to be separated and recovered from solution, thus hinder their practical application. Therefore, it is essential to immobilize photocatalysts on some substrates. However, after immobilization the photocatalytic activity of TiO2usually decreases[22].
In this study, polypyrrole-polyaniline copolymer/TiO2NT (PPy-PANI/TiO2NT) were synthesized by cyclic voltammetry (CV) technique. The morphology, crystalline structure, optical properties, and photocatalytic activities of PPy-PANI/TiO2NT were investigated. PPy-PANI/TiO2NT not only exhibits high photocatalytic activity under visible-light irradiation, but also can be recovered from solution easily.
2 Experimental
2.1 Materials
Titanium foil (99.8%, 0.3mm thick) was supplied by Aldrich (Milwaukee, WI). 4-nitrophenol (4NP) was purchased from Shanghai Fine Chemical Co., Ltd. (Shanghai, China). Sodium fluoride was purchased from Tianjin Tongxin Chemistry Co., Ltd. (Tianjin, China). Acetone was obtained from Shanghai Kefeng Chemistry Co., Ltd. (Shanghai, China). Sodium bisulfate, sulfuric acid, and absolute alcohol were supplied by Shantou Xilong Chemical Co., Ltd. (Shantou, China). Pyrrole (99% purity) were supplied by Aladdin Chemistry Co., Ltd. (Shanghai, China). Sodium sulfate was purchased from Chemical Plant of Hubei University (Wuhan, China). Aniline (99.5% purity) was purchased from Shanghai Zhanyun Chemistry Co., Ltd. (Shanghai, China). All the reagents were analytically pure, and were not puried. Water was purified by a Milli-Q water system (Bedford, USA).
2.2 Preparation of TiO2 nanotube arrays
Titanium foils (0.3mm thickness, 10mm×30mm) were cleaned ultrasonically in acetone and ethanol, respectively, followed by washed with deionized water. Ti foil was anodized using a DC power supply in an electrolyte of 0.5mol/L NaHSO4and 0.1mol/L NaF at 15V for 2h. The resulting TiO2NT was heated at 500℃ for 2h to form anatase.
2.3 Preparation of PPy-PANI/TiO2 NT, PANI/TiO2 NT and PPy/TiO2 NT
PPy-PANI/TiO2NT was prepared by CV scans between -0.4 and +1.0V for three scans with a scan rate of 50 mV/s. A three-electrode system was used with a carbon electrode as counter electrode, a KCl-saturated calomel electrode (SCE) as reference electrode, and TiO2NT as working electrode. The solution contains 0.5 mol/L H2SO4, 0.5mol/L Na2SO4, 0.05mol/L aniline, and 0.15mol/L pyrrole. After CV experiments, the films were washed and dried, and are denoted hereinafter as PPy-PANI/TiO2NT.
For comparison PANI/TiO2NT and PPy/TiO2NT composites were prepared and processed in parallel. Synthesis of PANI/TiO2NT was performed in the solution containing 0.2mol/L aniline, 0.5mol/L H2SO4and 0.5mol/L Na2SO4. PPy/TiO2NT was synthesized in the solution containing 0.2mol/L pyrrole, 0.5mol/L H2SO4and 0.5mol/L Na2SO4.
2.4 Characterization
The crystal structure of products was characterized using an automatic X-ray diffractometer with Cu Kα (Rigaku, Japan). The morphology and chemical elements of TiO2NT and PPy-PANI/TiO2NT were obtserved by scanning electron microscopy (SEM, Shimadzu, Japan) with energy dispersive spectroscopy (EDS, Shimadzn, Japan). Fourier transform infrared (FTIR) spectroscopy (Bruker, Germany) was also employed to do further characterization.
2.5 Photoelectrochemical measurements
All the electrochemical experiments were carried out on a CHI660C Electrochemical Workstation (Shanghai Chenhua Instrument Corporation, China). A three-electrode system was used with a carbon electrode as counter electrode, a KCl-saturated calomel electrode (SCE) as reference electrode, PPy-PANI/TiO2NT, PANI/TiO2NT and PPy/TiO2NT as working electrode, and 0.5mol/L Na2SO4as supporting electrolyte. A 300W xenon lamp with a 400nm cutofflter was utilized as visible-light source. The applied voltage was 0 V. All the potentials were respect to SCE.
2.6 Measurements of photocatalytic activities
Photocatalytic activity of PPy-PANI/TiO2NT, PANI/TiO2NT and PPy/TiO2NT was evaluated by degrading 10mg/L 4NP aqueous solution under the irradiation of a 300W xenon lamp with a 400nm cutofflter. 1 piece of PPy-PANI/TiO2NT, PANI/TiO2NT or PPy/TiO2NT was placed in 60mL of 4NP aqueous solution and stirred in the dark for 30min, the concentration of 4NP was determined and referred as the initial concentration C0. The 4NP solution was illuminated by visible light under continuous stirring. The 4NP solution was sampled at intervals of 15min, and was analyzed by a 754 UV-vis spectrometer at a wavelength of 320nm. The concentration of 4NP was determined by a calibration curve (Fig.1). The linearity range of 4NP was found between 0 and 12mg/L with correlation coefficient of 0.9998.
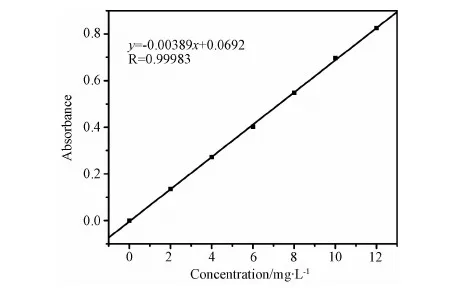
Fig.1 Calibration curve of 4NP solution
3 Results and discussion
3.1 XRD analysis
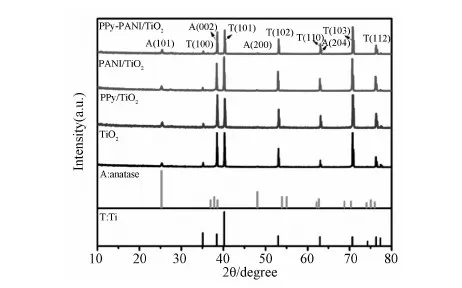
Fig.2 XRD patterns of TiO2 NT and PPy-PANI/TiO2 NT composites
XRD patterns of TiO2NT and PPy-PANI/TiO2NT are shown in Fig.2. The characteristic peaks of Ti were shown in the the XRD patterns as reference. Besides the characteristic peaks of Ti foil, the diffraction peak at 2θ=25.3° was indexed to (101) planes of anatase TiO2(PDF#21-1272), indicating that anatase TiO2was formed, and the PPy-PANI copolymer does not change the crystalline structure of TiO2.
3.2 Morphology of TiO2 NT and PPy-PANI/TiO2 composites
The SEM images of TiO2NT and PPy-PANI/TiO2NT composites were shown Fig.3. TiO2NT was composed of uniform, well-ordered, and vertically oriented TiO2nanotubes with pore size of about 90nm and wall thickness of about 20nm (Fig.3a). In case of PPy-PANI/TiO2NT, the walls of TiO2NT are unevenly covered PPy-PANI copolymer.
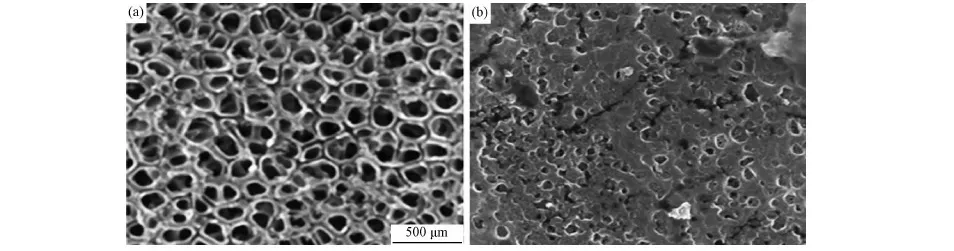
Fig.3 SEM images of (a) TiO2NT and (b) PPy-PANI/TiO2 NT composite
3.3 FTIR analysis
FTIR spectra of PANI/TiO2, PPy/TiO2and PPy-PANI/TiO2nanocomposites were shown in Fig.4. The peaks of PANI/TiO2at 1299 and 1520cm-1are attributed to C-N and C=C, respectively. In case of PPy/TiO2, the characteristic absorption peaks located at 1116 (C-N stretching mode), 1588 (C=C stretching mode) and 1027cm-1(pyrrole aromatic ring). The absorption peaks of PPy-PANI/TiO2located 1308 (C-N stretching mode), 1589 (C=C stretching mode) and 1121cm-1(pyrrole aromatic ring) indicate that the copolymer of pyrrole and aniline was deposited on TiO2NT successfully.
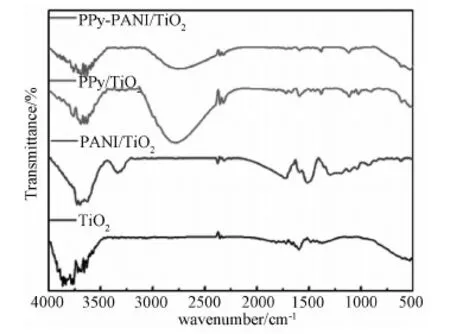
Fig.4 FT-IR spectra of PANI/TiO2, PPy/TiO2 and PPy-PANI/TiO2 nanocomposites
3.4 EDS analysis
Energy dispersion spectroscopy (EDS) was utilized to analyze the of element composition (Fig.5). Because TiO2NT was synthesized by anodization in a mixture solution containing NaHSO4and NaF, there are some residue elements, such as F, Na and S in the energy dispersion spectroscopy of PPy-PANI/TiO2NT. Moreover, N, O, Ti were present, confirming the formation of PPy-PANI/TiO2NT.
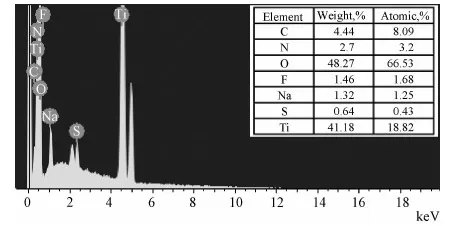
Fig.5 EDS of PPy-PANI/TiO2 NT composite
3.5 Photocurrent response
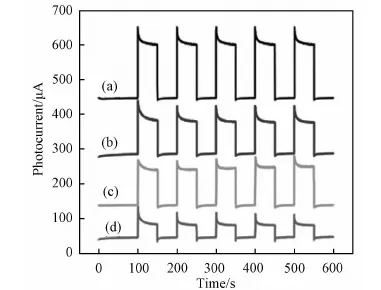
Fig.6 Transient photocurrent-time curves of (a) PPy-PANI/TiO2 NT, (b) PPy/TiO2 NT (c) PANI/TiO2 NT and (d) pure TiO2 NT electrodes under visible light irradiation
Fig.6 shows the photocurrent of TiO2NT and PPy-PANI/TiO2NT composites when light was turned on and off. The photocurrent of PPy-PANI/TiO2NT composites and TiO2NT was 52 and 21μA, respectively. The photocurrent results indicate the efficient separation and transfer of photogenerated electron-hole pairs in PPy-PANI/TiO2NT.
3.6 Photocatalytic activity of pure TiO2 NT, PPy/TiO2 NT, PANI/TiO2 NT and PPy-PANI/TiO2 NT under visible-light irradiation

Fig.7 Kinetic data for the degradation of aqueous 4NP on pure TiO2 NT, PPy/TiO2 NT, PANI/TiO2 NT and PPy-PANI/TiO2 NT under visible light irradiation
Fig.7 shows the photocatalytic activity of pure TiO2NT, PPy/TiO2NT, PANI/TiO2NT and PPy-PANI/TiO2NT by monitoring the degradation of 4NP solution. The regression curve of natural logarithm of normalized 4NP concentration versus reaction time was a straight line, demonstrating that the photocatalytic degradation of 4NP followed pseudo-rst order kinetics:
(1)
whereC0andCtare the concentration of 4NP (mg/L) at t=0 (min) and any timet(min), andkis the reaction rate constant (min-1). The rate constant of 4NP degradation over photocatalysts decreases in following order: PPy-PANI/TiO2NT>PPy/TiO2NT>PANI/TiO2NT>pure TiO2NT, indicating that PPy-PANI/TiO2NT composites exhibited the highest photocatalytic activity.
4 Conclusion
The PPy-PANI/TiO2NT was synthesized by cyclic voltammetry technique. The photocurrent of PPy-PANI/TiO2NT composites about is more than two times as high as that of the TiO2NT, indicating the efficient separation of photogenerated electron-hole pairs of PPy-PANI/TiO2NT. PPy-PANI/TiO2NT exhibited the highest photocatalytic activity among pure TiO2NT, PPy/TiO2NT and PANI/TiO2NT under visible-light irradiation.
Reference
[1] Deng F, Liu Y, Luo X, et al. Enhanced Photocatalytic Activity of Bi2WO6/TiO2Nanotube Array Composite under Visible Light Irradiation[J]. Separation and Purification Technology, 2013, 120: 156~161.
[2] Bhatti Z I, Toda H, Furukawa K. P-Nitrophenol Degradation by Activated Sludge Attached on Nonwovens[J]. Water Research, 2002, 36(5): 1135~1142.
[3] Gu Y, Zhang Y, Zhang F, Wei J, Wang C, Du Y, Wei C. Investigation of Photoelectrocatalytic Activity of Cu2O Nanoparticles for P-Nitrophenol using Rotating Ring-Disk Electrode and Application for Electrocatalytic Determination[J]. Electrochimica Acta, 2010, 56(2): 953~958.
[4] Wang Y, Bai X, Pan C, He J, Zhu Y. Enhancement of Photocatalytic Activity of Bi2WO6Hybridized with Graphite-like C3N4[J]. Journal of Materials Chemistry, 2012, 22(23): 11568~11573.
[5] Dong W, Lee C W, Lu X, et al. Synchronous Role of Coupled Adsorption and Photocatalytic Oxidation on Ordered Mesoporous Anatase TiO2-SiO2Nanocomposites Generating Excellent Degradation Activity of Rhb Dye[J]. Applied Catalysis B: Environmental, 2010, 95(3): 197~207.
[6] Luo X, Deng F, Min L, et al. Facile One-step Synthesis of Inorganic-framework Molecularly Imprinted TiO2/WO3Nanocomposite and its Molecular Recognitive Photocatalytic Degradation of Target Contaminant[J]. Environmental Science & Technology, 2013, 47(13): 7404~7412.
[7] Wang H, Duan J, Cheng Q. Photocatalytically Patterned Tio2Arrays for On-plate Selective Enrichment of Phosphopeptides and Direct MALDI MS Analysis[J]. Analytical Chemistry, 2011, 83(5): 1624~1631.
[8] Wang P,Yap P S,Lim T T. C-N-S Tridoped TiO2for Photocatalytic Degradation of Tetracycline Under Visible-light Irradiation[J]. Applied Catalysis A: General, 2011, 399(1): 252~261.
[9] Jin Y, Zhao G, Wu M, et al. In Situ Induced Visible-light Photoeletrocatalytic Activity from Molecular Oxygen on Carbon Aerogel-supported TiO2[J]. The Journal of Physical Chemistry C, 2011, 115(20): 9917~9925.
[10] 景茂祥, 顏遠瞻, 平昱航, 李立康. 介孔二氧化鈦微球光催化劑的結(jié)構(gòu)與性能[J]. 材料科學與工程學報, 2014, 32(1): 5~9.
[11] Deng F, Li Y, et al. Preparation of Conductive Polypyrrole/TiO2Nanocomposite Via Surface Molecular Imprinting Technique And Its Photocatalytic Activity Under Simulated Solar Light Irradiation[J]. Colloids and Surfaces A: Physicochemical And Engineering Aspects, 2012, 395: 183~189.
[12] Zhang X, Li X, Shao C, et al. One-Dimensional Hierarchical Heterostructures of In2S3Nanosheets on Electrospun TiO2Nanofibers with Enhanced Visible Photocatalytic Activity[J]. Journal of Hazardous Materials, 2013, 260: 892~900.
[13] Deng F, Min L, Luo X, Wu S, Luo S. Visible-light Photocatalytic Degradation Performances and Thermal Stability Due to the Synergetic Effect of TiO2with Conductive Copolymers of Polyaniline and Polypyrrole[J]. Nanoscale, 2013, 5(18): 8703~8710.
[14] Shang X, Li B, Li C, et al. Preparation and Enhanced Visible Light Catalytic Activity of TiO2Sensitized with Benzimidazolone Yellow H3G[J]. Dyes and Pigments, 2013, 98(3): 358~366.
[15] Nuraje N, Su K, Yang N, Matsui H. Liquid/Liquid Interfacial Polymerization to Grow Single Crystalline Nanoneedles of Various Conducting Polymers[J]. ACS Nano, 2008, 2(3): 502~506.
[16] Hong S Y, Park S M. Electrochemistry of Conductive Polymers 36. Ph Dependence of Polyaniline Conductivities Studied by Current-sensing Atomic Force Microscopy[J]. The Journal of Physical Chemistry B, 2005, 109(19): 9305~9310.
[17] Karim M R, Lim K T, Lee C J, et al. Synthesis of Core-shell Silver-Polyaniline Nanocomposites by Gamma Radiolysis Method[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2007, 45(24): 5741~5747.
[18] Karim M R, Lee H W, Cheong I W, et al. Conducting Polyaniline-Titanium Dioxide Nanocomposites Prepared by Inverted Emulsion Polymerization[J]. Polymer Composites, 2010, 31(1): 83~88.
[19] 王彥紅, 王德松, 等. Ppy/TiO2納米復(fù)合材料的制備及光催化活性[J]. 材料科學與工程學報, 2008, 26(2): 284~287.
[20] Wang D, Wang Y, Li X, et al. Sunlight Photocatalytic Activity of Polypyrrole-TiO2Nanocomposites Prepared By ‘in Situ’Method [J]. Catalysis Communications, 2008, 9(6): 1162~1166.
[21] Li J, Zhu L, Wu Y, et al. Hybrid Composites of Conductive Polyaniline and Nanocrystalline Titanium Oxide Prepared Via Self-Assembling and Graft Polymerization[J]. Polymer, 2006, 47(21): 7361~7367.
[22] Souzanchi S, Vahabzadeh F, et al. Performance of an Annular Sieve-plate Column Photoreactor Using Immobilized TiO2on Stainless Steel Support for Phenol Degradation [J]. Chemical Engineering Journal, 2013, 223: 268~276.
[23] Xu Z, Yang W, Li Q, Gao S, Shang J. Passivated N-P Co-Doping of Niobium and Nitrogen into Self-Organized TiO2Nanotube Arrays for Enhanced Visible Light Photocatalytic Performance[J]. Applied Catalysis B: Environmental, 2014, 144: 343~352.
[24] Muramatsu Y, Jin Q, Fujishima M, Tada H. Visible-Light- Activation of TiO2Nanotube Array by the Molecular Iron Oxide Surface Modification[J]. Applied Catalysis B: Environmental, 2012, 119: 74~80.
[25] 譚志謀, 王慧潔, 楊杭生,張孝彬. 陽極氧化法制備二氧化鈦納米管陣列的形貌[J]. 材料科學與工程學報, 2013, 31(3): 390~394+385.
[26] 王月勤, 陶杰, 何娉婷. 二氧化鈦納米管上電沉積羥基磷灰石[J]. 材料科學與工程學報, 2007, 25(2): 249~252.

