Characteristics and Degradation Mechanism of Fomesafen
Tao Bo, Qiao Yu-xin, Ma Cheng-yi, Shao Bai-hui, and Jie Bai
College of Agricultural, Northeast Agricultural University, Harbin 150030, China
Introduction
Fomesafen {5-[2-chloro-4-(trifluoromethyl)phenoxy]-N-(methylsulfonyl)-2-nitrobenzamide} is a selective herbicide that may be applied post emergence for control or suppression of broadleaf weeds, grasses and sedges in soybean (Zhanget al., 2014).Fomesafen is exploited by British Zeneca Company.Its target enzyme is protoporphyrin oxidase (Jacobset al., 1996;Willows, 2003; Jacobset al., 1991).Fomesafen was first registered in China in 1988, when it was widely applied to soybean, peanut and oil crops to control broadleaf weeds because of its high efficiency, low toxicity and good selectivity (Caquetet al., 2005;Peacheyet al., 2012; Baileyet al., 2003).Recently,adjustments to the planting structure and development of the herbicide industry have led to increased field usage of fomesafen.However, this compound has a long residual effect on the soil, and its halflife is almost 100 days.Because this is the longest half-time of diphenyl ether herbicide varieties, it can exert phytotoxic effects, leading to reduce or complete loss of harvests (Liuet al., 2008; Rauchet al., 2007; Khorramet al., 2015).Therefore, it is important to study the characteristics and degradation mechanisms of fomesafen.Degradation of herbicides in soil primarily occurs through hydrolysis, in which microbial degradation plays an important role (Fenget al., 2012).Lianget al.(2009) studied theBacillusstrain ZB-1 of fomesafen degradation product is N-[4-(4-(trifluoromethyl) phenoxy}-2-benzoyl] acetamide.The degradation products are N-[4-(4-trifluoromethyl) phenoxy}-2-benzoyl]acetamide, 3-(4-(trifluoromethyl) phenoxy)-6-cyano-2,4-diene aldehyde, 3-(4-(trifluoromethyl) phenoxy)-5-carboxyl-2 pentene, 4-3 methyl acetylene ether,respectively.However, no studies have investigated the microbial mechanism of fomesafen degradation yet.Therefore, the characteristics and mechanisms of microbial degradation of fomesafen were investigated.
Materials and Methods
Reagents
Fomesafen (98%) was provided by Jiangshu Changqing Pesticide Company.
Bacteria 2 strain (B2S) was cloned byAspergillus flavus(TZ1985) and it was provided by the Pesticide Lab in Northeast Agricultural University, Harbin,China.
Growth and degradation characteristics of B2S fomesafen degrading bacteria
Preparation of the degradation seed liquid of B2S:Luria-Bertani liquid medium was inoculated with B2S.Cultured for 16-24 h.Sampled adjust OD600=0.8.The strain concentration was used as the seed liquid of B2S in the following steps.
Briefly, 100 mL of Luria-Bertani liquid medium was added to 250-mL Erlenmeyer flasks and inoculated with 1%, 3%, 5%, 7% and 5% access activation good seed liquid of B2S.Samples were then incubated at 20, 25, 30, 35 and 40°C, respectively.In addition, the initial pH of the medium was adjusted to 5.0, 6.0, 7.0,8.0 and 9.0, respectively.Initial glucose concentrations of 0%, 0.1%, 0.3%, 0.5%, 0.7% and 0.9%, respectively were also investigated.Samples were adjusted to 0,5, 10, 25 and 50 mg · L-1fomesafen, respectively.Each processing set was repeated in triplicate and the solutions were cultured for 72 h, while shaking at 200 r · min-1, and then measured the absorbance values by spectrometer to obtain the growth rate of B2S.Finally, the degradation rate of fomesafen was determined by HPLC.
Evaluation of fomesafen degradation products produced by B2S
1.One hundred mL LB liquid medium was placed into 250-mL Erlenmeyer flasks.
2.Fomesafen was added to give a final concentration of 10 mg · kg-1.
3.Inoculated with 5% seed liquid of B2S after activation.
4.Each processing set was repeated in triplicate and the solutions were cultured for 72 h, while shaking at 200 r · min-1.Set temperature to 35℃.
5.Sampling 5 mL seed liquid of B2S in a bacteriafree operating environment was transferred to 10-mL centrifuge tubes at 0, 12, 24, 36, 48, 60 and 72 h,respectively.
6.Centrifuged under the condition of 15 000 r · min-1,4℃ for 10 min.
7.Got 3.0 mL supernatant and added 1.0 g of solid sodium chloride to it.
8.When the solid sodium chloride dissolved, added 9 mL (3+3+3 mL) methylene chloride extraction three times, merged methylene chloride layer.
9.Added 0.5 g of anhydrous sodium sulfate and dried, transferred supernatant to a new centrifugal tube.Dried by blowing nitrogen, with the capacity to the mobile phase 3 mL.
10.Filtered by 0.22-um microporous membrane filter and reserved.
Test conditions:
Chromatographic conditions: 20 μL aliquots of samples were applied to a Waters Xbridge C18column(2.1×150 mm, 5 μm) at a flow rate of 0.3 mL · min-1,while maintaining a column temperature of 30℃.
Mass spectrometry conditions: electrospray ionization (ESI) ion source; scanning in positive and negative ion mode; scanning range, 50-800 m · z-1,gasification chamber temperature, 370℃; nitrogen as auxiliary gas and scavenging gas, helium as collision gas (Table 1).
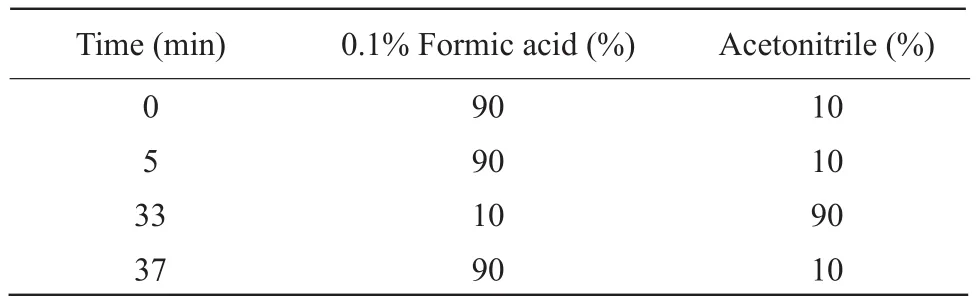
Table 1 Gradient elution conditions
Based on HPLC analysis, the appropriate reaction liquid was selected to investigate extraction of de-gradation products of fomesafen.According to the interpretation of result of HPLC-MS, the degradation products and metabolic pathways were inferred.
Results
Growth and degradation characteristics of B2S
The optimum cultivation conditions for fomesafen degradation bacterium B2S were as the followings:temperature 35℃; inoculation quantity 5%; pH 5.0;glucose content 0.5% and fomesafen concentration 10 mg · L-1.Under these conditions, the degradation rate of fomesafen was 99.2% within 72 h (Figs.1-6).
Degradation products of fomesafen generated by B2S bacteria
After fomesafen was degraded by B2S strain bacteria,multiple degradation products were produced in amounts that varied with degradation time.Fomesafen degradation products of liquid chromatography/mass spectra and the corresponding mass spectrometry data were obtained by LC-MS (Fig.7).The structure of the fomesafen degradation products was determined using the data shown in Table 2.
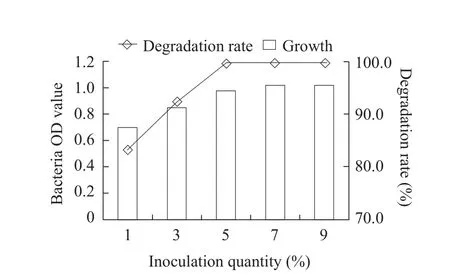
Fig.1 Effect of quantity of inoculation on growth of B2S and fomesafen degradation rate
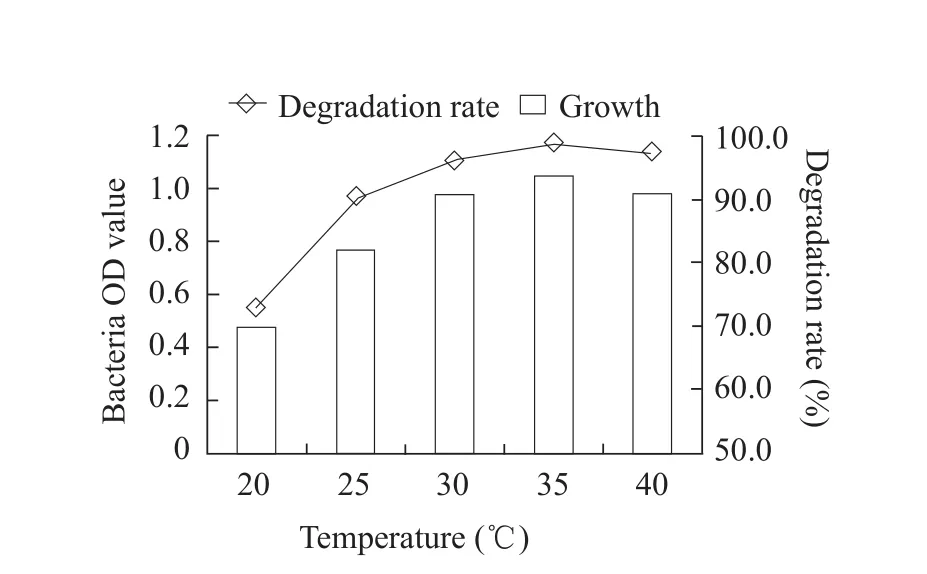
Fig.2 Effect of temperature on growth of B2S and fomesafen degradation rate
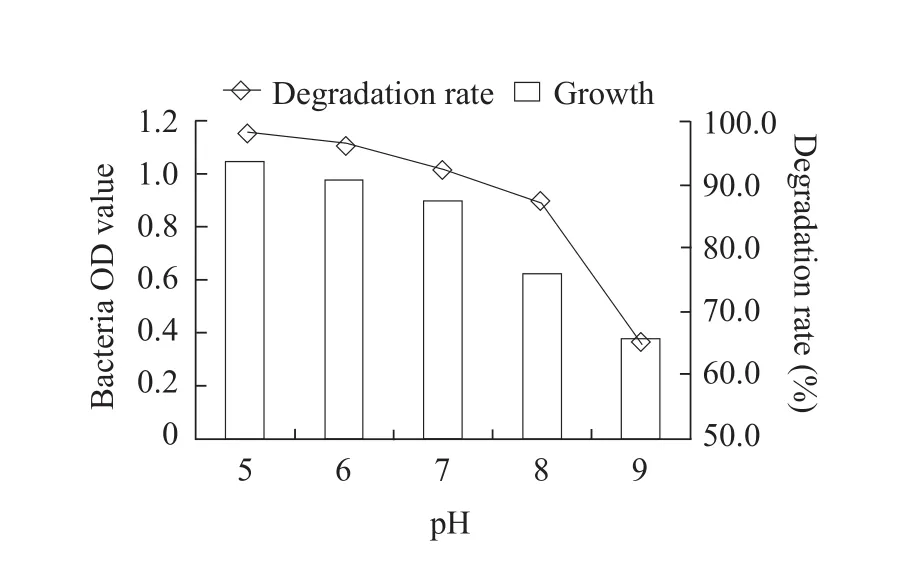
Fig.3 Effect of culture medium pH on growth of B2S and fomesafen degradation rate
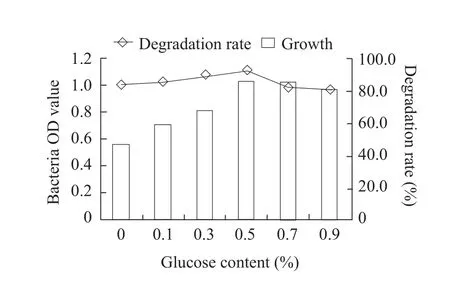
Fig.4 Effect of glucose content on growth of B2S and fomesafen degradation rate
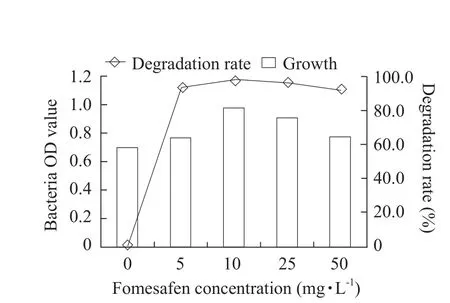
Fig.5 Effect of fomesafen concentration on growth of B2S and fomesafen degradation rate
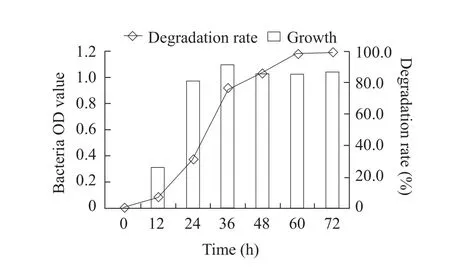
Fig.6 Effect of time on growth of B2S and fomesafen degradation rate
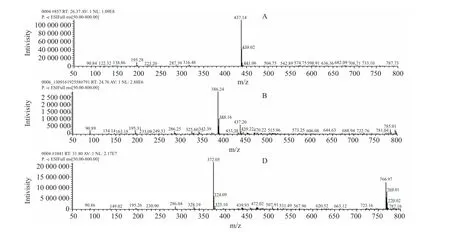
Fig.7 Mass spectra of degradation product A, B and D
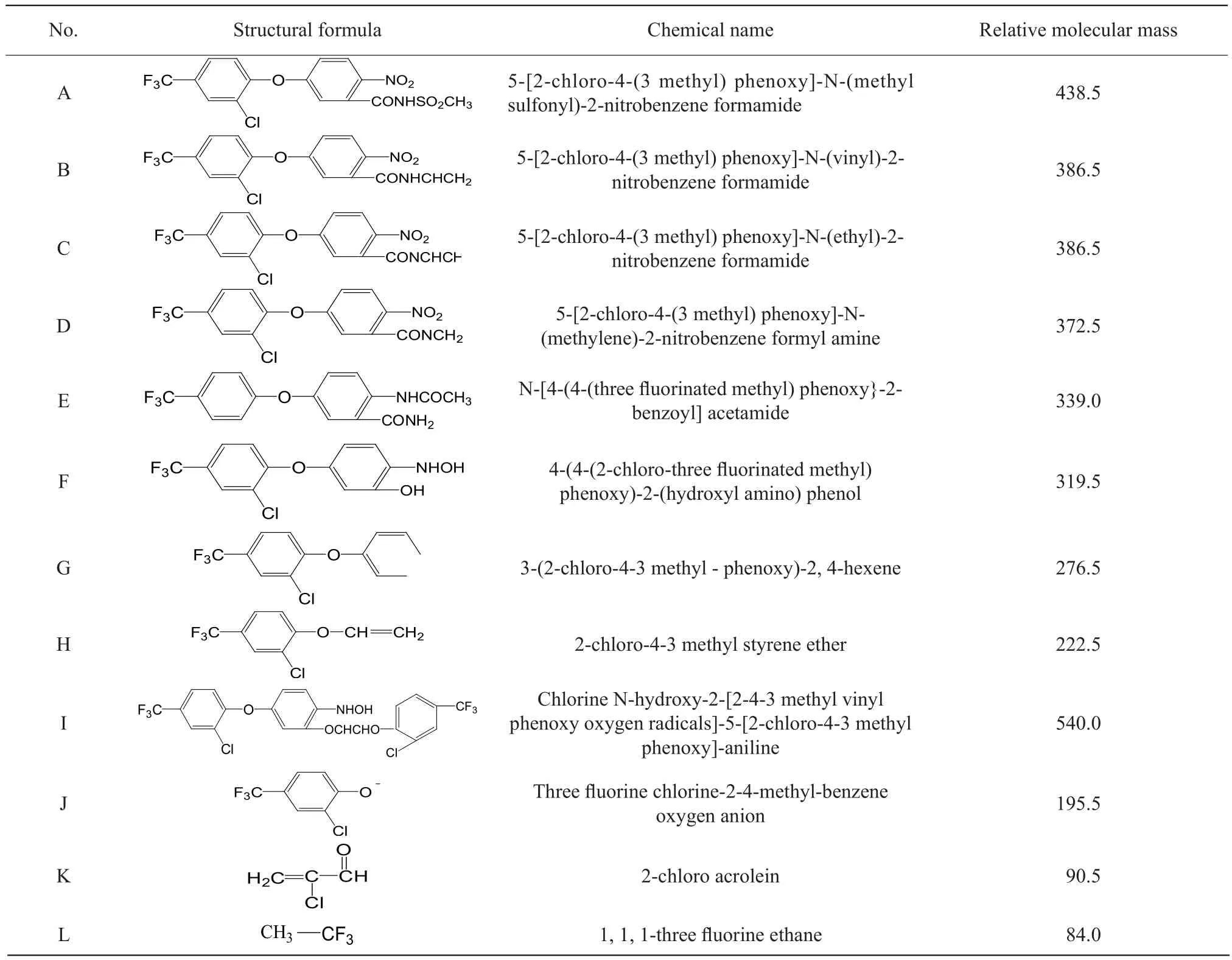
Table 2 Structure of fomesafen and its metabolites
Mechanism of bacterial degradation of fomesafen
According to mass spectral data of fomesafen degradation products and the hydrolysis products of fomesafen, analyzed the research to fomesafen degradation products of degrading bacteria by Lianget al(2009).
The pathway of fomesafen degradation by B2S is shown in Fig.8.
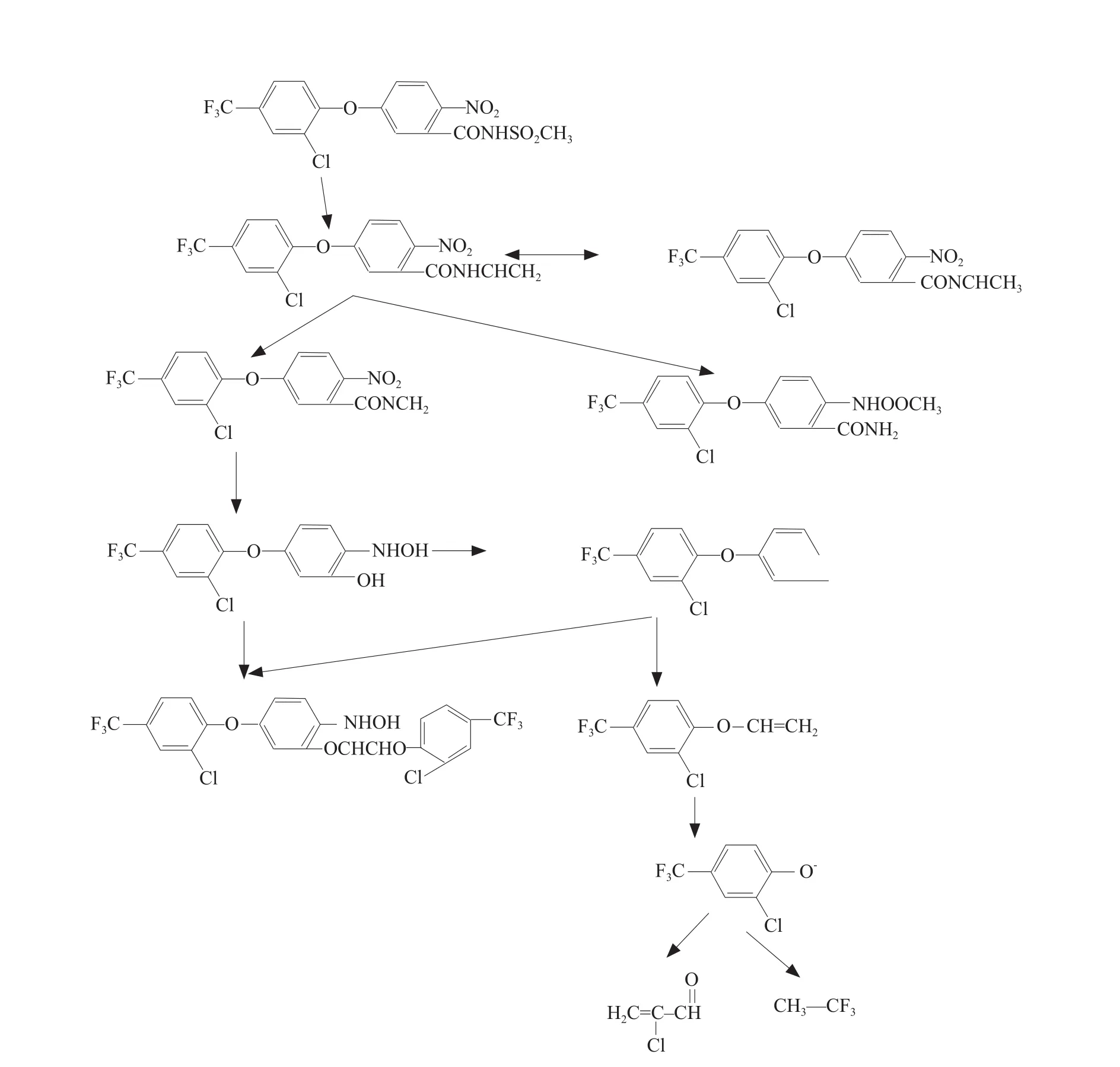
Fig.8 Pathway of fomesafen degradation by B2S
Discussion
Fomesafen soil pollution is mainly controlled through microbial degradation, soil degradation and vegetation degradation, with microbial degradation being the most effective, safest and convenient method for its remediation identified to date.In 2001, studies were conducted to identify bacteria capable of degrading diphenyl ether herbicide.Hiratsukaet al.(2001)investigated the degradation of nitrogen by whiterot fungi.Younget al.(2008) found that RW1 could degrade chlomethoxyfen, nitrogen and oxyfluorfen.Zhanet al.(2011) found that the fungus,AspergillusflavusTZ1985, could degrade fomesafen.Moreover,previous studies revealed thatLysinibacillussp.(Yanget al., 2012),Fusiformissp.(Ahmedet al., 2007),Pseudomonassp.(Fenget al., 2012),Chryseomonasluteolasp.(Smith-Greenier and Adkins, 1996) andStenotrophomonas acidaminiphila(Assihet al., 2002;Yanget al., 2014; Laganàet al., 2000) could degrade fomesafen.The bacteria screened from the soil was polluted with herbicides, and this bacteria could degrade diphenyl ether herbicide.Under the optimum conditions, B2S was able to degrade 71.6% of 10 mg · L-1fomesafen within 36 h and nearly 100%within 72 h, which is much higher than that reported for other strains to date.
There were three primary products of fomesafen hydrolysis: 4-[2-chloro-4-(trifluoromethyl) phenoxy]-nitrobenzene, 4-[2-chloro-4-(trifluoromethyl) phenoxy]-phenoxy iron and 2-chloro-4-trifluoromethyl styrene ether.In this experiment, 11 degradation products were identified, one of which was concordant with the results of Lianget al.(2011), and another was in accordance with that reported by Aldoet al(2000).Based on the products identified after various degradation periods, the mechanism of fomesafen degradation by B2S was then speculated.
Conclusions
In the present study, B2S could degrade fomesafen effectively in sorts of liquid medium.The effects of growth and degradation characteristics of B2S were examined.Eleven metabolites of fomesafen degradation by strain B2S have been found.The reaction might be caused by the fomesafen reduction and acetylation of nitro and dechlorination of fomesafen.In this study, the high removal rate of fomesafen in the strain B2S indicated that the strain was useful in bioremediation for contamination with fomesafen in the environment.
The authors wish to thank all the colleagues who assisted in this research and provided technical advice.We would like to thank Editage(www.editage.com) for English language.
Ahmed I, Yokota A, Yamazoe A,et al.2007.Proposal ofLysinibacillus boronitoleransgen.nov.sp.nov., and transfer ofBacillus fusiformistoLysinibacillus fusiformiscomb.Nov.andBacillus sphaericustoLysinibacillus sphaericuscomb.nov.International Journal of Systematic and Evolutionary Microbiology, 57(5): 1117-1125.
Aldo L, Giovanna F, Laura F,et al.2000.Determination of diphenylether herbicides and metabolites in natural waters using highperformance liquid chromatography with diode array tandem mass spectrometric detection.Analytica Chimica Acta, 414: 79-94.
Assih E A, Ouattara A S, Thierry S,et al.2002.Stenotrophomonas acidaminiphilasp.nov., a strictly aerobic bacterium isolated from an up flow anaerobic sludge blanket (UASB) reactor.International Journal of Systematic and Evolutionary Microbiology, 52(2):559-568.
Bailey W A, Wilson H P, Hines T E.2003.Weed control and snap bean (Phaseolus vulgaris) response to reduced rates of fomesafen.Weed Technology, 17(2): 269-275.
Caquet T, Deydier-Stephan L, Lacroix G,et al.2005.Effects of fomesafen, alone and in combination with an adjuvant, on plankton communities in freshwater outdoor pond mesocosms.Environmental Toxicology and Chemistry/SETAC, 24: 1116-1124.
Feng Z Z, Li Q F, Zhang J,et al.2012.Microbial degradation of fomesafen by a newly isolated strainPseudomonas zeshuiiby-1 and the biochemical degradation pathway.Journal of Agricultural and Food Chemistry, 60(29): 7104-7110.
Hiratsuka N, Wariishi H, Tanaka H.2001.Degradation of diphenyl ether herbicides by the lignin-degrading basidiomyceteCoriolus versicolor.Applied Microbiology and Biotechnology, 57(4):563-571.
Jacobs J M, Jacobs N J, Duke S O.1996.Protoporphyrinogen destruction by plant extracts and correlation with tolerance to protoporphyrinogen oxidase-inhibiting herbicides.Pesticide Biochemistry and Physiology, 55(1): 77-83.
Jacobs J M, Jacobs N J, Sherman T D,et al.1991.Effect of diphenyl ether herbicides on oxidation of protoporphyrinogen to protoporphyrin in organellar and plasma membrane enriched fractions of barley.Plant Physiology, 97(1): 197-203.
Khorram M S, Wang Y, Jin X,et al.2015.Reduced mobility of fomesafen through enhanced adsorption in biochar-amended soil.Environmental Toxicology and Chemistry, 34(6): 1258-1266.
Liu H, Jiang G M, Zhuang H Y,et al.2008.Distribution, utilization structure and potential of biomass resources in rural China: with special references of crop residues.Renewable and Sustainable Energy Reviews, 12(5): 1402-1418.
Liang B, Lu P, Li H,et al.2009.Biodegradation of fomesafen by strainLysinibacillussp.ZB-1 isolated from soil.Chemosphere, 77(11):1614-1619.
Laganà A, Fago G, Fasciani L,et al.2000.Determination of diphenylether herbicides and metabolites in natural waters using highperformance liquid chromatography with diode array tandem mass spectrometric detection.Analytica Chimica Acta, 414(1/2): 79-94.
Peachey E, Doohan D, Koch T.2012.Selectivity of fomesafen based systems for preemergence weed control in cucurbit crops.Crop Protection, 40: 91-97.
Rauch B J, Bellinder R R, Brainard D C,et al.2007.Dissipation of fomesafen in New York State soils and potential to cause carryover injury to sweet corn.Weed Technology, 21(1): 206-212.
Smith-Greenier L L, Adkins A.1996.Degradation of diclofop-methyl by pure cultures of bacteria isolated from Manitoban soils.CanadianJournal of Microbiology, 42(3): 227-233.
Willows R D.2003.Biosynthesis of chlorophylls from protoporphyrin IX.Natural Product Reports, 20(3): 327-341.
Yang F, Zhou Y, Yin L,et al.2014.Microcystin-degrading activity of an indigenous bacterial strainStenotrophomonas acidaminiphilaMCLTH2 isolated from Lake Taihu.PLoS ONE, 9(1).
Yang L L, Huang Y, Liu J,et al.2012.Lysinibacillus mangiferahumisp.nov., a new bacterium producing nematicidal volatiles.Antonie van Leeuwenhoek,International Journal of General and Molecular Microbiology, 102(1): 53-59.
Young S K, Young J L, Kim J H.2008.Metabolism of nitrodiphenyl ether herbicides by dioxin-degrading bacteriumSphingomonas wittichiiRW1.Journal of Agricultural and Food Chemistry, 56(19):9146-9151.
Zhang Q, Zhu L, Wang J,et al.2014.Effects of fomesafen on soil enzyme activity, microbial population, and bacterial community composition.Environmental Monitoring and Assessment, 186(5):2801-2812.
Zhan H X, Ren H L, Jiang L X,et al.2011.Separation, identification and growth characters of herbicide fomesafen degrading-fungi.Crops, 2: 40-44.
 Journal of Northeast Agricultural University(English Edition)2018年1期
Journal of Northeast Agricultural University(English Edition)2018年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Operational Performance Evaluation of Corn Processing Industry Technological Innovation Alliance Based on Survey Data in Heilongjiang Province
- Spatial and Temporal Variation in Water Productivity and Grain Water Utilization Assessment of Heilongjiang Province, Northeast China
- Analysis of Process of Microwave Puffing Blueberry Snacks
- Stability Analysis of a Lignocellulose Degrading Microbial Consortium
- Pharmacokinetics of Milbemycin Oxime in Dogs Following Its Intravenous and Oral Administration
- Comparative Research on Facultative Anaerobic Cellulose Decomposing Bacteria Screened from Soil and Rumen Content and Diet of Dairy Cow
