Isotopic Effects on Stereodynamics of the C++H2→ CH++H Reaction?
Lu Guo(郭璐),Yun-Fan Yang(楊云帆),,2Xiao-Xing Fan(范曉星),Feng-Cai Ma(馬鳳才),,?and Yong-Qing Li(李永慶),?Department of Physics,Liaoning University,Shenyang 0036,China
2State Key Laboratory of Molecular Reaction Dynamics,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China
1 Introduction
The C++H2→CH++H reaction,a prototype ionmolecule reaction studied extensively in recent decades,has been playing a significant role in the dynamics associated with simple chemical reactions and development of potential energy surface(PES).[1?2]This reaction has been investigated by several experimental groups and has gradually become the point of much theoretical and experimental activities.[3]The methylidyne cation CH+is a familiar molecular ion in cometary and planetary atmospheres and diffuse interstellar clouds.[4]Ever since the methylidyne cation CH+was found for the first time in the diffuse interstellar medium by Herzberg and Douglas,it has been detected in all kinds of circumstelar and interstellar circumstances.[5]In spite of it was the first cation ever justified in the interstellar space,[6]there are only a few results of vector properties about the cation CH+.[7]
First of all,in the past 60 years,CH+was only discovered through itsA1Π ?X1Σ+electronic band system observed in assimilation toward mildly reddened OB stars,so tracking the diffuse interstellar medium in the vicinity of sun.The Herschel Space Telescope and the Infrared Space Observatory have since expanded the scope of research,the study of far-infrared rotational spectrum of CH+that formerly cannot be discovered at the ground surface on account of the high opacity of the atmosphere.In the new scope of spectral,CH+has been discovered in assimilation from the diffuse interstellar medium in the internal Galactic disk,and emission from denser gas in the planetary nebulae NGC 7027,the proto planetary disk HD 100546 and the Orion Bar photo dissociation region.We can draw a conclusion that the methylidyne cation CH+is omnipresent in the whole interstellar matter.[5]
In this work,we have applied a new kind of ground state PES forThen using quasi classical trajectory(QCT)method to study the C++H2reaction and fixed the collision energy with a value is 40 kcal/mol.The total reactive cross section can be written as a function of collision energy has been studied by Maie[8]and Lindemann et al.[9]Moreover,an extensive study has been carried out to analyze the properties of vectors related to orientation and alignment of the product CH+.[10?13]Jones et al.have investigated the rotational and vibrational of the CH+produced.[14]In this sense we feel that C++H2will play a role comparable to that which F+H2has played[15?21]for neutral A+BC reactions.The paper is arranged as follows:A brief review the main content of the theoretical methodologies in Sec.2.In Sec.3,the calculation results of QCT method are discussed in detail.In the end,the conclusion is presented in Sec.4.
2 Theory
2.1 Vector Correlations
Recently,the reaction C++H2and its isotopic variants have received extensive attention.But,most stud-ies in the previous work are to explore the scalar nature of the reaction.In order to understand the dynamics of the C++H2reaction and its isotopic substituted reactions fully,it is important to study not only their scalar properties,but also their vector properties.Vector properties,such as velocities and angular momentum,possess not only magnitudes that can be directly related to translational and rotational energies,but also well defined directions.Only by understanding together the above properties can the fullest picture of the scattering dynamics be obtained.
The differential reaction cross sections(DCSs)dσ/dωiis the most common vector relation,which is used to describe the relative velocity vectors(k,k′)of the reactant and product.The vectors k′and k are respectively represented product relative velocity and reactant relative velocity,and the vector j′is product rotational angular momentum.The k ? k′,k ? j′and k ?k′? j′vector correlations can provide kinetic information on both orientation and alignment of the product.[22?23]
As shown in Fig.1,the center of mass(CM)coordinates has been employed at the present work.Here,k is along thez-axis and they-axis is perpendicular to thex-zplane including k and k′vectors,which is the scattering plane for delineating preferably dynamic properties of title reaction.The scattering angles between k and k′are represented by the symbolsθt,?randθrdenote the azimuthal angles and polar of the product rotational angular momentum j′.[24?27]
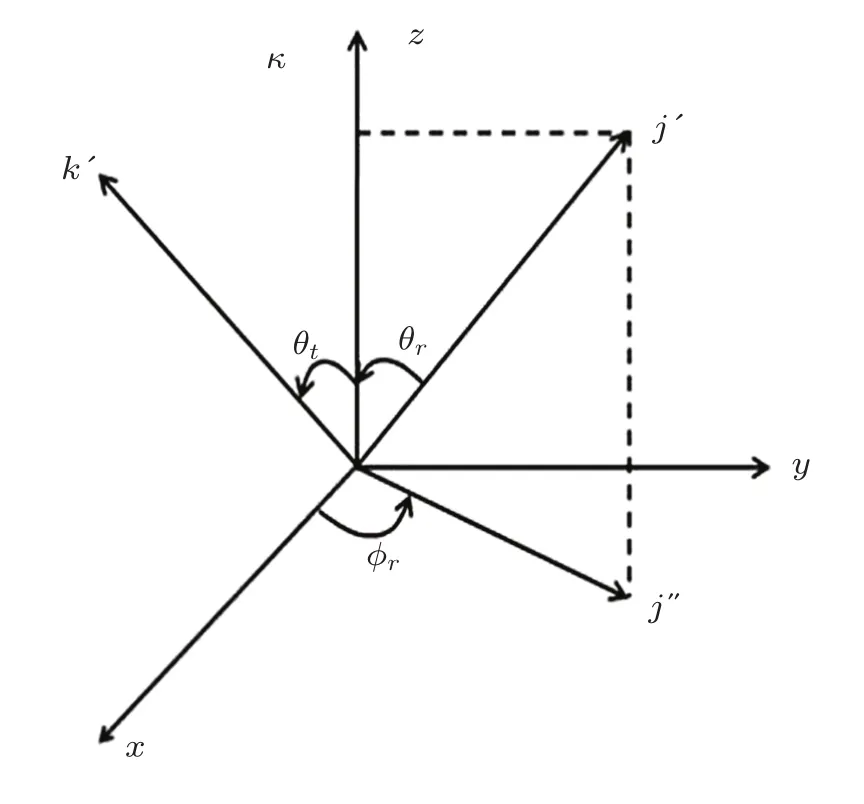
Fig.1 Center of mass coordinate system used to describe the k ? k′? j′correlations.
The P(θr)distribution corresponding to the vector correlation k?j′is represented a series of Legendre polynomials[28?37]
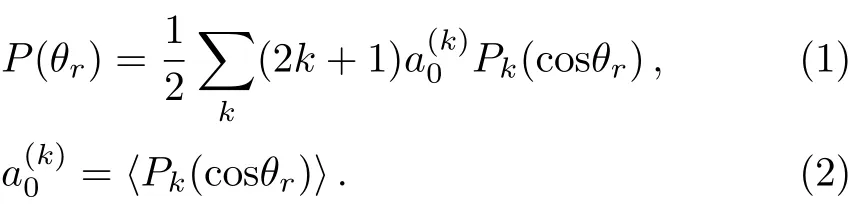
The angle?ris used to describe the k ? k′? j′vector correlation,P(?r)distribution function could be represented as a Fourier expansion[28?37]
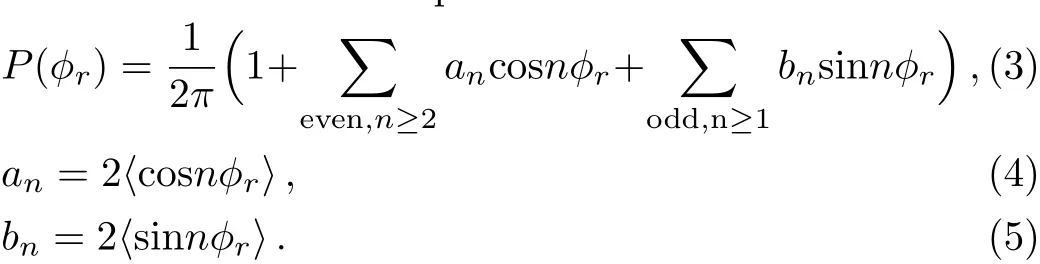
In the process of calculation,P(?r)is expanded up ton=24 that gets a better convergence result.
Usually,using joint probability density function of anglesθrand?rto define the direction of j′,P(θr,?r)could be represented as,
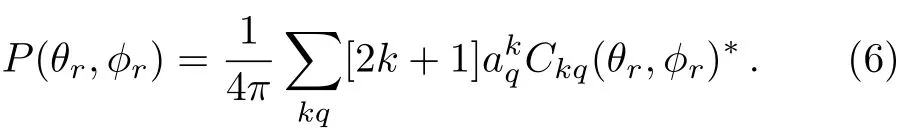
In this work,P(θr,?r)is expanded up tok=7,which is suffcient for good convergence.
2.2 Quasi-classical Trajectory Calculation
The effects of isotope substitution on stereodynamic properties for the reactions
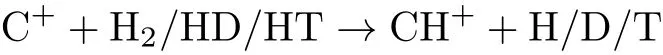
have been studied applying a QCT method occurring on many kinds PESs.The common method that the QCT calculation is the same as the previous work,and the classical Hamilton′s equation is integrated numerically for move in three-dimensional space.Lately,many theoretical and experimental investigations of the reactions C++H2/HD/HT→CH++H/D/T and its isotope substitution has been carried out at the relatively high collision energies close to the threshold of reaction.
In order to study the different dynamic information based on the ground state ofPES and fixed the collision energy with a value is 40 kcal/mol in this paper.A total of 20000 trajectories have been run for each of the C++H2and its isotope substitution reaction.The classical motion equations have been integrated using a time step of 0.1 fs,with H2molecule and the center of mass of the C+atom initially separated by 15.0?A.[38]
2.3 Potential Energy Surface
For the reaction C++H2/HD/HT→CH++H/D/T,the QCT calculation has occurred on the ground state ofPES.[39]And it has been built based on a leastsquares fitting to precise high level ab initio MRCI(Q)energy,applying AV6Z basis set to calculate.The various topographical features of the PES have been carefully compared with other realistic PESs available in the literature.This new PES is described by a relatively simple equation and has a smooth and correct behavior at the whole configuration space.
All known stationary structures and dissociation energies from the new PES can be obtained,and are in good agreement with corresponding laboratorial value.Specifically,the potential for CH+obtained by the new PES has an accurate dissociation energy and equilibrium geometry compared with the corresponding experimental value.Based on the new PES,we perform a time dependent scattering wave packet calculations for the reaction C++H2→CH++H.The results of integral cross sections and rate coeffcient have been shown to be in good agreement with effective theoretical and experimental value.On the basis of the above,the new PES can be recommended for dynamics studies of any type.
3 Results and Discussion
3.1 Differential Cross-Sections
The DCSs provides a good chance to investigate the most common vector correlation in the product and reagent relative velocity k ? k′. Fixing the collision energy with a value is 40 kcal/mol to observe the isotope variants on DCS distribution for the reaction C++H2/HD/HT→CH++H/D/T.As illustrated in Fig.2,product mainly presented the forward scattering and backward scattering,in addition,we can find that if the target molecule H2is substituted by HD and HT,the forward scattering phenomenon becomes more and more obvious,backward scattering phenomenon gradually weakened and finally the reaction is mainly governed by forward scattering.So we can get such a conclusion:under the same collision energy while the mass of the target molecules increases(H2→HD→HT),the forward scattering gradually strengthen,backward scattering is gradually weakened, finally the reaction is mainly dominated by forward scattering,which is consistent with a capture mechanism,where the abstraction reaction is dominant.
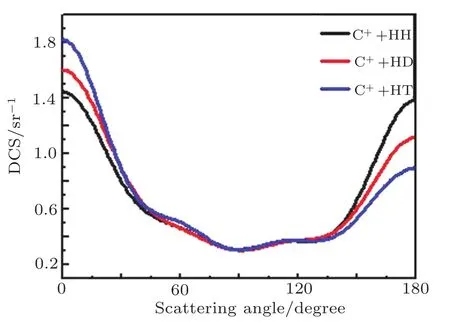
Fig.2 The DCS for the reactions C++H2/HD/HT→CH++H/D/T of the collision energies of 40 kcal/mol plotted as a function of scattering angle.
3.2 k?j′Vector Correlation
The calculated distributions of P(θr)for the reaction C++H2/HD/HT→CH++H/D/T with the collision energies of 40 kcal/mol is plotted in Fig.3.The P(θr)distribution,describing the reactant relative velocity vector k and the product rotational angular momentum vector j′the correlation,and the distribution P(θr)is symmetric aboutθr=90°with showing maximum at 90°.It can be concluded that j′is distributed with cylindrical symmetry in the product scattering frame and the direction of j′is preferentially perpendicular to the k direction.
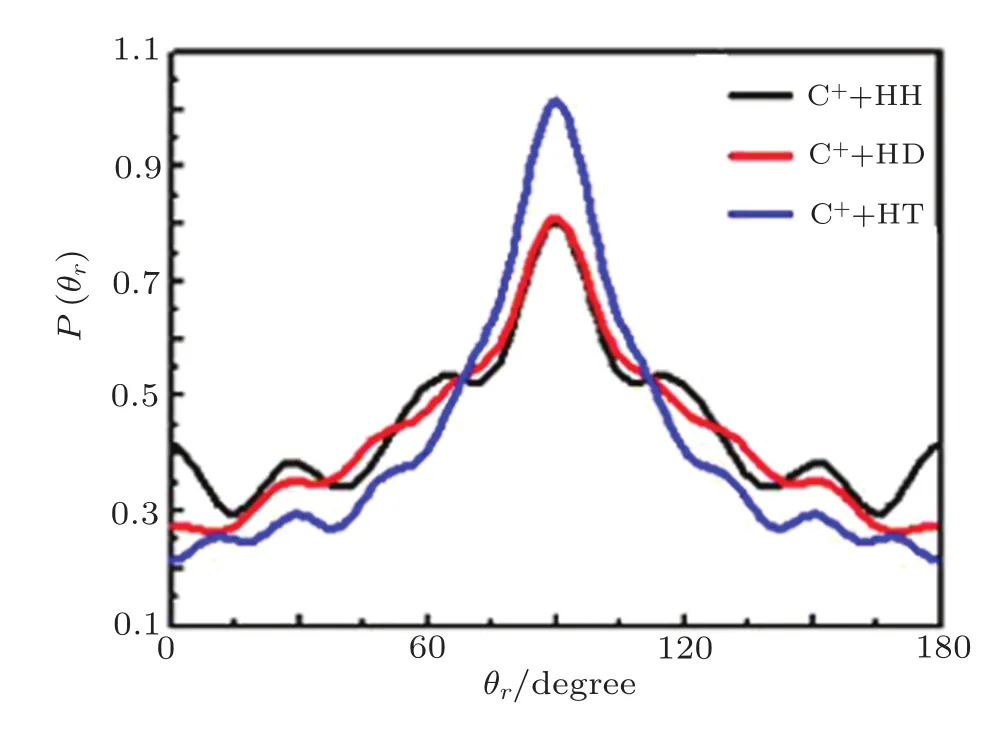
Fig.3 Distribution of P(θr)reflecting the k ? j′correlation.
For the reaction A+BC→AB+C,the k?j′correlation is sensitive to two factors:one is mass factor cos2β=mAmC/(mA+mB)(mB+mC),another is the structure features of PES.So we can find from the Fig.3,with the increase of the mass of the target molecule atθr=90°that peak gradually increased,the reaction C++H2has a minimum value and the reaction C++HT has a maximum value.These results imply that the rotational angular momentum of the product is weakly influenced by the mass of attacking ion,but it is strongly enhanced while the mass of the target molecule increases.So in the molecular reaction dynamics,alignment degree of the product is proportional to the quality of the reactants.
3.3 k?k′?j′Vector Correlation
Figure 4 presents the P(?r)distribution of j′direction for product with the collision energy of 40 kcal/mol,which mainly describing the k?k′?j′vector correlation. The P(?r)distribution of the three reactions is asymmetry with respect to?r=π,this asymmetric distribution implies the strong polarization of product molecule rotational angular momentum.The peak of the P(?r)distribution at?rangle about 3π/2 describes a tendency to left-handed product rotation while the peak at?rangle aboutπ/2 describes a tendency to right-handed product rotation.The P(?r)distribution in?r=π/2 and?r=3π/2 has extreme value,this results suggest that the product rotational angular momentum j′is mainly aligned along they-axis in the center of mass coordinates.Due to there are different extreme values at?r=π/2 and?r=3π/2,we can know that j′not only has alignment effect but also has orientation effect.As Fig.4 shows,for the three reactions,the peak at?r=π/2 is slightly higher than the one at?r=3π/2,illustrating the j′not only aligned along the direction that perpendicular to the relative velocity of reactants,also oriented the positive direction of they-axis.It should be noted that with the increase of the atomic mass,the peaks of P(?r)distributions become more and more narrow,which indicates the rotation of the product molecule has been changing from the “out-of-plane” reaction mechanism to the “in-plane”reaction mechanism.In turn,as depicted in Fig.5,for the reaction C++H2,the P(?r)distributions display a distinct“V” shaped splitting around?r=90°,which predicts a dynamic enhancement of the degree of orientation along they-axis.
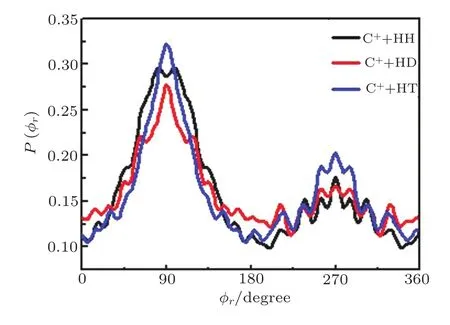
Fig.4 Dihedral angle distribution of P(?r),describing the k ?k′?j′correlation.
According to instantaneous collision model of the reaction A+BC→AB+C,the product molecular rotation angular momentum j′is written as[22,38,40]
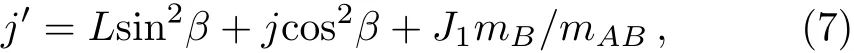
where
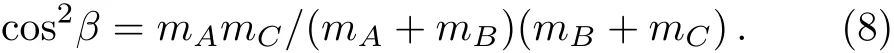
And
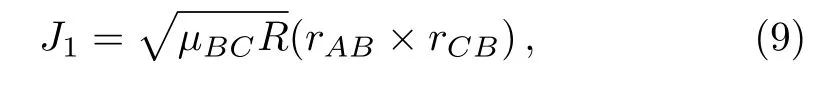
where L is reactant molecule orbital angular momentum andJ1is reactant molecule rotational angular momentum,cos2βsignify the mass factor,R is the repulsive energy andμBCis the reduced mass of BC molecule,rABandrCBare unit vectors that B atom points to A atom and C atom,respectively.During the chemical bond forming and breaking for the title reactions,the termLsin2β+Jcos2βin Eq.(7)is symmetrical about the k?k′scattering plane.However,J1mB/mABtends to a preferred direction because of the effect of repulsive energy R,which causes the orientation of the product.
In order to show the angular momentum polarization clearly,the distribution of P(θr,?r)has been calculated(shown in Fig.5).The angular momentum polarization in the form of polar plot of the distribution of P(θr,?r)is also depicted in Fig.5,which represents the scattering angle average of the full distribution of P(ωt,ωr).In the Fig.5,it is easy to see that the P(θr,?r)distribution at(π/2,π/2)has an obvious distribution.The distributions of the P(θr,?r)peaks at(π/2,π/2)are in good accordance with the distributions of P(θr)and P(?r).
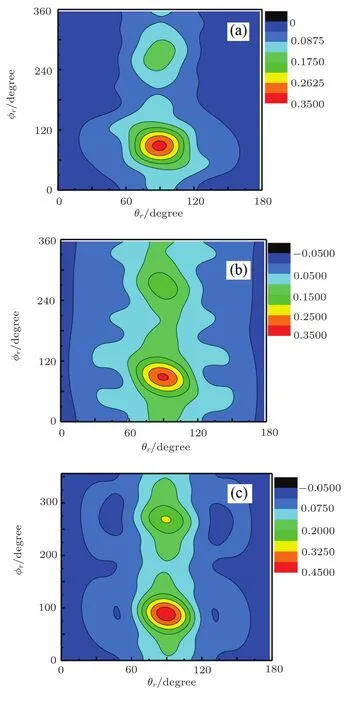
Fig.5 Contours of the joint angular distribution P(θr,?r)averaged over all scattering angles of C++H2/HD/HT→CH++H/D/T.(a)H2;(b)HD;(c)HT.
4 Conclusions
In the present work, the stereodynamics of C++H2/HD/HT→CH++H/D/T isotope substitution reaction is investigated applying QCT method based on the ground statePES.The 20000 trajectories have been run with the collision energy 40 kcal/mol to investigate the isotope effect on the product alignment and orientation.The results of the calculated DCSs demonstrate that the k?k′angular distribution is dominated by forward scattering,and the degree of the scattering will increase with the increase of reactant molecular quality.Three angle distribution functions P(θr),P(?r),and P(θr,?r)with different target molecules H2/HD/HT are calculated at the same collision energy,it is shown that the product rotational angular momentum is not only aligned,but also oriented along the direction perpendicular to the scattering plane.The calculated distribution of P(θr,?r)indicates that the CH+product is preferentially polarized perpendicular to the scattering plane and the reactions are mainly dominated by “in-plane” mechanism.In summary,the isotope effect is obviously influence on the properties of stereodynamics.
[1]S.Sakai,S.Kato,and K.Morokuma,J.Chem.Phys.75(1981)5398.
[2]M.Gonzalez,A.Aguilar,and J.Virgili,Chem.Phys.Lett.113(1985)187.
[3]D.H.Liskow,C.F.Bender,and H.F.Schaefer III,J.Chem.Phys.61(1974)2507.
[4]E.Picazzio,A.D.Almeida,S.M.Andrievskii,I.K.Churyumov,and I.V.Luk′yanykProceedings of Asteroids,ed.B Warmbein,ESA Publications Division,Berlin,Germany(2002)pp.713–716.
[5]A.Zanchet,B.Godard,N.Bulut,O.Roncero,P.Halvick,and J.Cernicharo,Astrophys.J.766(2013)766.
[6]A.E.Douglas and G.Herzberg,Astrophys.J.94(1941)381.
[7]R.Warmbier and R.Schneider,Phys.Chem.Chem.Phys.13(2011)10285.
[8]W.B.Maier II,J.Chem.Phys.46(1967)4991.
[9]E.Lindemann,L.C.Frees,R.W.Rozett,and W.S.Koski,J.Chem.Phys.56(1972)1003.
[10]C.R.Iden,R.Liardon,and W.S.Koski,J.Chem.Phys.54(1971)2757.
[11]C.R.Iden,R.Liardon,and W.S.Koski,J.Chem.Phys.56(1972)851.
[12]B.H.Mahan and T.M.Sloane,J.Chem.Phys.59(1973)5661.
[13]E.Herbst,R.L.Champion,and L.D.Doverspike,J.Chem.Phys.63(1975)3677.
[14]C.A.Jones,K.L.Wendell,and W.S.Koski,J.Chem.Phys.66(1977)5325.
[15]T.P.Schafer,P.E.Siska,J.M.Parson,F.P.Tully,Y.C.Wong,and Y.T.Lee,J.Chem.Phys.53(1970)3385.
[16]J.C.Polanyi and K.B.Woodall,J.Chem.Phys.57(1972)1574.
[17]R.D.Coombe and G.C.Pimentel,J.Chem.Phys.59(1973)1535.
[18]J.T.Muckerman,J.Chem.Phys.57(1972)3388.
[19]N.C.Blais and D.G.Truhlar,J.Chem.Phys.58(1973)1090.
[20]G.G.Schatz,J.M.Bowman,and A.Kuppermann,J.Chem.Phys.58(1973)4023.
[21]C.F.Bender,S.V.O’Neil,P.K.Pearson,and H.F.Schaefer,Science 176(1972)1412.
[22]M.D.Chen,K.L.Han,and N.Q.Lou,Chem.Phys.Lett.357(2002)483.
[23]M.D.Chen,M.L.Wang,K.L.Han,and S.L.Ding,Chem.Phys.Lett.301(1999)303.
[24]G.M.Mcclelland and D.R.Herschbach,J.Phys.Chem.A 83(1979)1445.
[25]J.D.Barnwell,J.G.Loeser,and D.R.Herschbach,J.Phys.Chem.A 87(1983)2781.
[26]Z.X.Duan,W.L.Li,and M.H.Qiu,J.Chem.Phys.136(2012)144309.
[27]X.L.Chi,J.F.Zhao,Y.J.Zhang,F.C.Ma,and Y.Q.Li,Chin.Phys.B 24(2015)053401.
[28]M.L.Wang,K.L.Han,and G.Z.He,J.Phys.Chem.A 102(1998)20204.
[29]M.L.Wang,K.L.Han,and G.Z.He,J.Chem.Phys.109(1998)5446.
[30]M.D.Chen,K.L.Han,and N.Q.Lou,Chem.Phys.283(2002)463.
[31]M.D.Chen,K.L.Han,and N.Q.Lou,J.Chem.Phys.118(2003)4463.
[32]X.Zhang and K.L.Han,Int.J.Quantum Chem.106(2006)1815.
[33]K.L.Han,G.Z.He,and N.Q.Lou,J.Chem.Phys.105(1996)8699.
[34]D.H.Cheng,J.C.Yuan,and M.D.Chen,J.Phys.Chem.A 1(2014)55.
[35]S.L.Liu and Y.Shi,Chin.Phys.Lett.27(2010)123103.
[36]Y.J.Ding and Y.Shi,Comput.Theor.Chem.963(2011)306.
[37]Y.Y.Zhang,Y.Shi,T.X.Xie,M.M.Jin,and Z.Hu,Chin.Phys.B 22(2013)083402.
[38]X.H.Li,M.S.Wang,Ilaria Pino,C.L.Yang,and J.C.Wu,Phys.Chem.Chem.Phys.12(2010)7943.
[39]Y.Q.Li,P.Y.Zhang,and K.L.Han,J.Chem.Phys.142(2015)124302.
[40]X.H.Li,M.S.Wang,Ilaria Pino,C.L.Yang,and L.Z.Ma,Phys.Chem.Chem.Phys.11(2009)10443.
 Communications in Theoretical Physics2017年5期
Communications in Theoretical Physics2017年5期
- Communications in Theoretical Physics的其它文章
- Anharmonic Properties of Aluminum from Direct Free Energy Interpolation Method?
- Effects of Interfaces on Dynamics in Micro-Fluidic Devices:Slip-Boundaries’Impact on Rotation Characteristics of Polar Liquid Film Motors?
- Controlling Thermodynamic Properties of Ferromagnetic Group-IV Graphene-Like Nanosheets by Dilute Charged Impurity
- Elastic Deformation Analysis on MHD Viscous Dissipative Flow of Viscoelastic Fluid:An Exact Approach
- Entropy Generation Analysis in Convective Ferromagnetic Nano Blood Flow Through a Composite Stenosed Arteries with Permeable Wall
- Borromean Windows for Three-Particle Systems under Screened Coulomb Interactions?
