The Function of β Zeolite for Enhancing the Propylene Yield in FCC Process
Ouyang Ying; Luo Yibin; Shu Xingtian
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
1 Introduction
Propylene is one of the most important petrochemical raw materials. However there has been a constant and huge gap between market demand and supply of propylene in the world, especially in China having large capacity of chemical industry. Maximizing the propylene production from FCCU has been proved to be effective solution for bridging the gap between demand and supply of propylene by using the Y and ZSM-5 zeolites as catalysts.
At present, the study on catalytic materials for maximizing propylene yield is mainly focused on the ZSM-5 zeolite with other catalytic materials rarely involved[1-2]. In this study, we hope to find some new catalytic materials to replace or to work in conjunction with ZSM-5 for maximizing the propylene yield in FCC unit.
The hydrocarbon composition in catalytic cracking process is very complex, since the hydrocarbon molecules with different sizes exist in the reaction feed.Because of the difference of pore size and geometry,the molecular sieve catalytic materials with different
pore structures have different accessibility to different hydrocarbon molecules, and can thus affect the cracking performance of the catalysts. The Y, β and ZSM-5 zeolites with different pore structures have their respective characteristics in the cracking reaction performance,especially the ability to produce propylene. This paper will further study the differences between these materials,and explore the cracking reaction performance and the ability to produce propylene by compounding several molecular sieves. We hope each molecular sieve can bring into full play its respective advantages and generate the synergistic effect to maximize the selectivity and yield of propylene.
2 Experiments
The ultra-stable Y zeolite, the modified β zeolite and the modified ZSM-5 zeolite were aged at 800 °C for 4 h in steam, respectively. The acidity of these samples was characterized by the NH3-TPD analysis. The evaluation ofcatalytic activity and selectivity of the sample was carried out in a microreactor unit using hexane, decane, and light diesel fuel or VGO as feedstocks.
3 Results and Discussion
The Y, ZSM-5, and β zeolites have been used as catalytic materials in FCC process currently, the topological structures of which have their own effects on their catalytic performance. The Y zeolite is the main zeolite used in FCC process. Among these zeolites, the Y zeolite has supercages (having a size of 1.25 nm) with the largest window (0.74 nm × 0.74 nm), which is a favorable room for cracking the heavy oil molecules into gasoline and diesel fractions. With crossed tenmembered ring channels, the ZSM-5 zeolite has been widely used in various types of catalytic reactions thanks to its shape selectivity, and is the most successful FCC additive for producing propylene. The β zeolite is distinctive on topological structure and has become a new catalytic material, which is applied extensively with good prospects. The pore structure characteristics of these three kinds of zeolites are summarized in Table 1. The data of Table 1 indicate that the channels connecting each other are separated by symbol “? ” and the dimension of the channel system is represented by the numbers of symbols “*”.
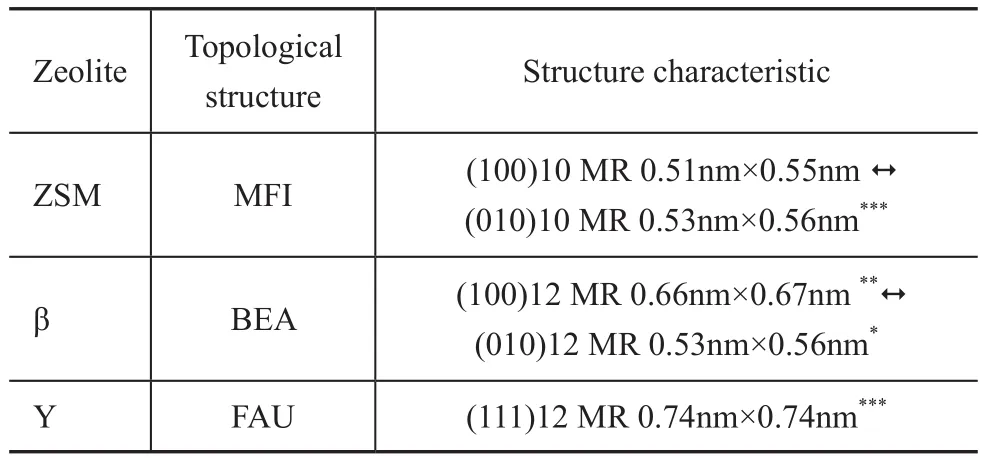
Table 1 Pore structure characteristics of zeolites
In this study, we select three industrial zeolites provided by the Sinopec Catalyst Co., LTD. The ultra-stable Y zeolite sample is prepared by the hydrothermal method.The β zeolite sample is a kind of β zeolite modified by phosphorus and transition metal. The ZSM-5 sample is a kind of ZSM-5 zeolite modified by phosphorus and transition metal. The physical and chemical properties of zeolite samples are listed in Table 2.
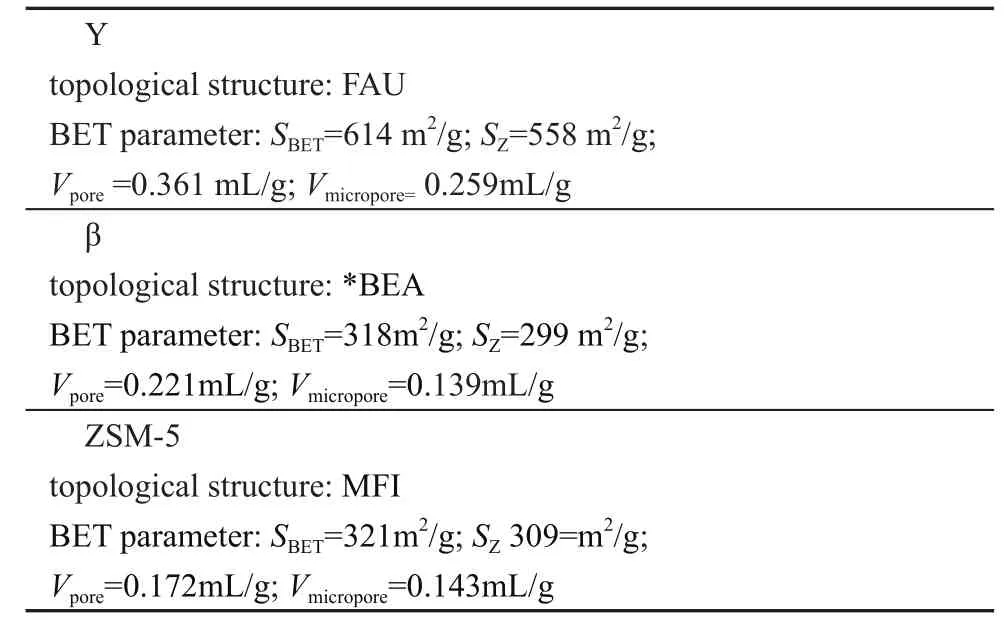
Table 2 Physical and chemical properties of zeolites
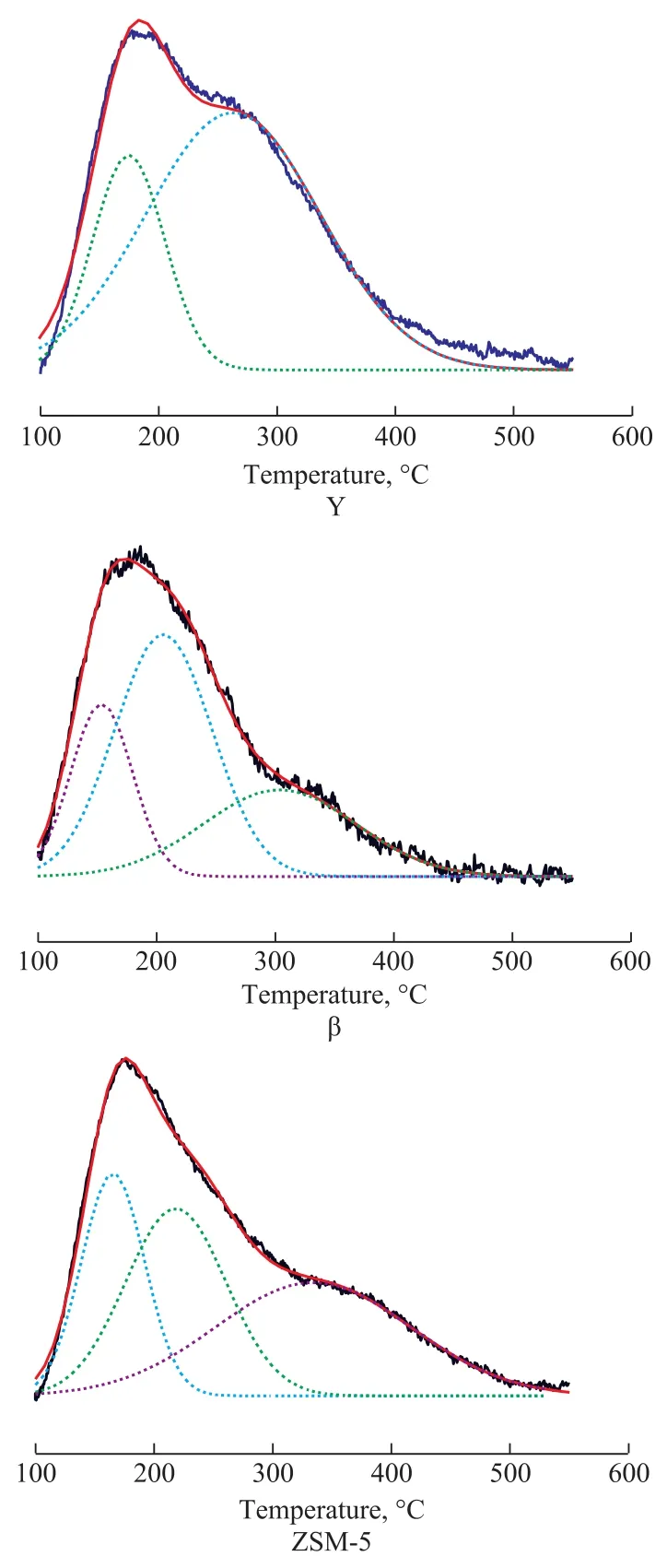
Figure 1 NH3-TPD spectra of zeolites
The zeolite samples mentioned above were hydrothermally treated in a fixed-bed device, and were then characterized by the NH3-TPD analysis. The results are listed in Figure 1.Figure 1 shows that the Y zeolite has two desorption peaks.One formed at below 200 °C corresponds to the weak acid sites, and the other one formed at between 200 °C and 300°C corresponds to the medium-strong acid sites. Besides that, the β and ZSM-5 zeolites have desorption peaks formed at above 300 °C corresponding to strong acid sites.We can get the acid number of different acid sites by decon via the Gaussian fitting. The data are listed in Table 3.It can be seen that the ZSM-5 zeolite has the least amount of acids, however its acid strength and strong acid sites are the highest among three samples. In addition, the acid amount of Y zeolite is the largest, but its acid strength is mainly composed of weak acid sites and medium-strong acid sites, because no strong acid sites are found in the desorption peak at 300 °C. The acidity of β zeolite is between that of Y zeolite and ZSM-5 zeolite.
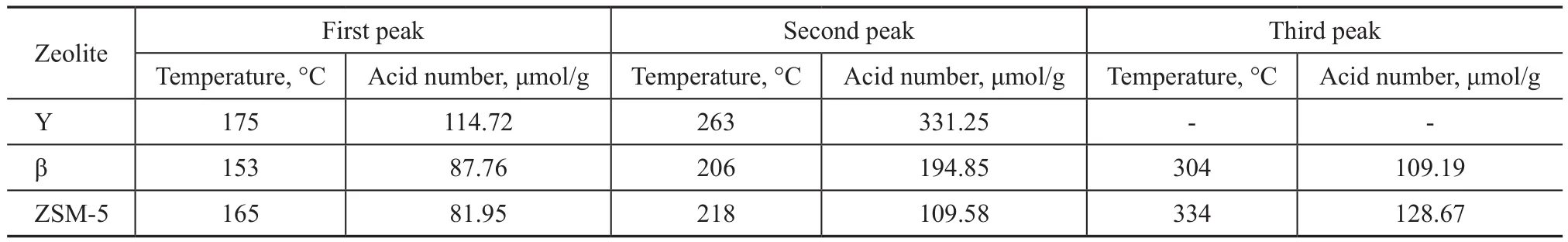
Table 3 Surface acid characterization of the zeolites
Since the property of β zeolite is between that of Y zeolite and ZSM-5 zeolite in terms of the channel structure and acidity, the cracking behavior of different reactants over these three zeolites was studied.
Hexane, decane and diesel were selected as feedstocks to evaluate the cracking behavior of zeolites respectively.The zeolites were subjected to ageing at 800 °C for 4 h in 100% of steam, and were tested in a micro-reactor unit.
The result of hexane cracking over three zeolite samples is listed in Table 4. It can be seen from Table 4 that upon cracking of hexane over these three zeolite samples the ZSM-5 zeolite achieved a highest conversion, propylene yield and product selectivity.Hexane molecules are small enough in size (with a kinetic diameter of 0.38 nm) to diffuse easily into the channels of these three kinds of zeolites to access the acid sites. However, the hexane cracking reaction needs strong acid sites because the activation energy of hexane cracking is high. According to acid analysis of three kinds of zeolites, ZSM-5 zeolite has the strongest acid centers so that it can crack hexane molecular most easily. The β zeolite assumes the second place in terms of its acid strength. Although the number of acid density of Y zeolite is the biggest, but most of them are weak acid sites and medium-strong acid sites and there are nearly no strong acid sites, so that the hexane cracking reaction with high activation energy is difficult to take place over the Y zeolite.
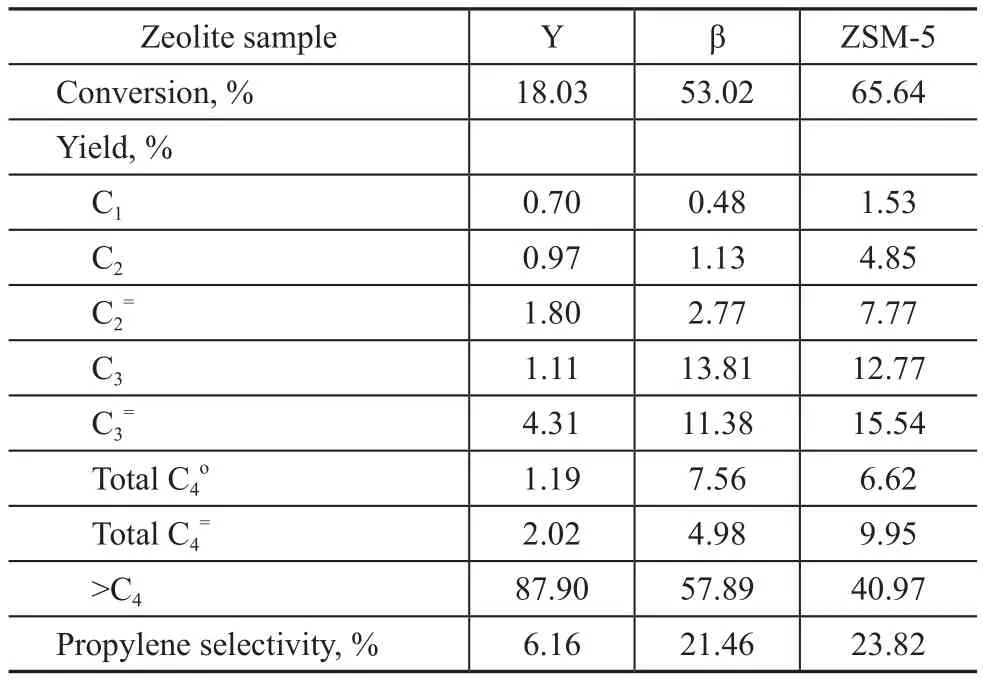
Table 4 Test results of the aged zeolite samples in hexane cracking
Secondly, the test results of decane cracking over three zeolite samples are listed in Table 5.
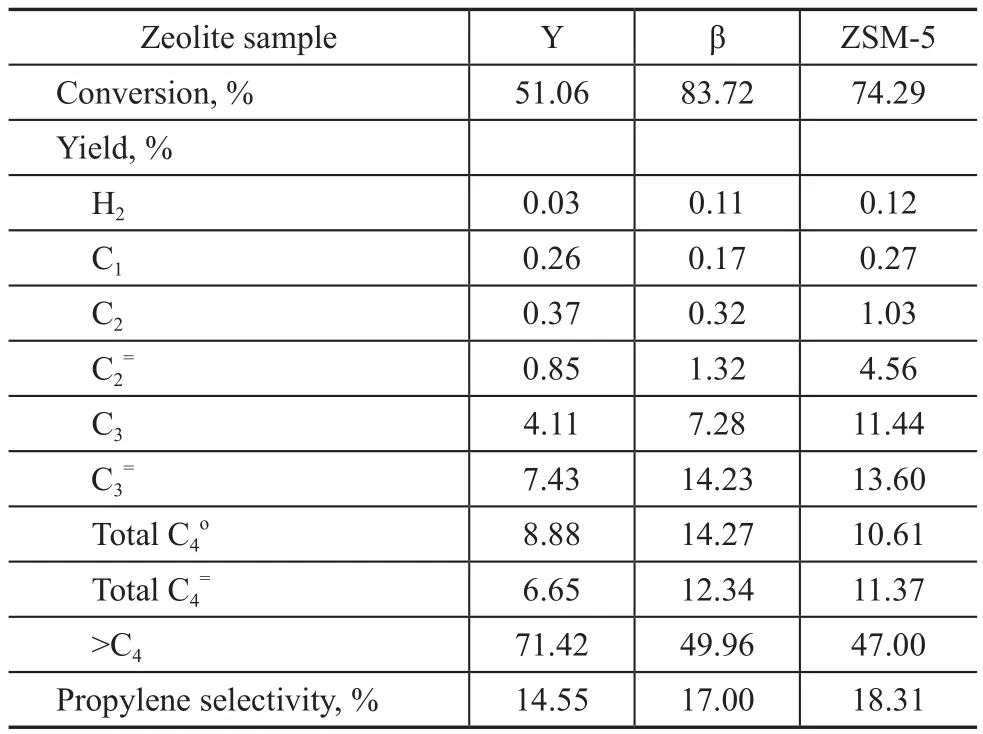
Table 5 Test results of the aged zeolite samples in decane cracking
In general, the cracking activity of chain alkanes increased with the chain length. Compared with hexane, decane can be cracked more easily over acid sites. However,decane has bigger molecular size to restrict its diffusion into zeolite channels, and thus the ZSM-5 zeolite shows lower conversion in decane cracking reaction under the in fl uence of diffusion constraints. As regards the decane cracking, the β zeolite shows the highest conversion and propylene yield. But the ZSM-5 zeolite has the highest propylene selectivity because of its shape selection function.
Thirdly, the test results of diesel cracking over three zeolite samples are listed in Table 6.
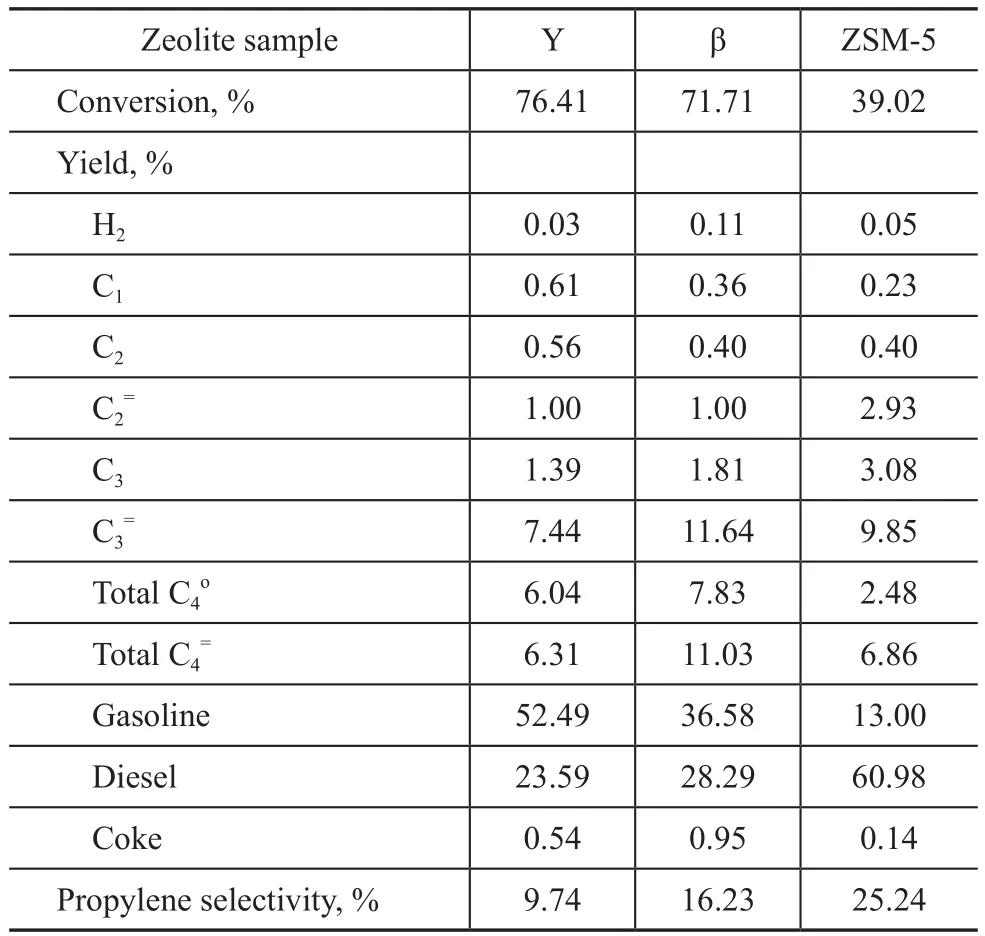
Table 6 Test results of the aged zeolite samples in diesel cracking
During diesel cracking, the diffusion limitation significantly affects the reactions involving larger size of raw molecules, so that the Y zeolite with the biggest pore size has the highest conversion but it has the lowest propylene yield and selectivity. The conversion over zeolite sample decreases in the following order:Y zeolite >β zeolite >ZSM-5 zeolite. It is same as the order of pore size in zeolite. At the same time, we also can see that the propylene selectivity over zeolite sample decreases in the following order: ZSM-5 zeolite >β zeolite >Y zeolite. The propylene selectivity is related to the features of zeolite sample in pore structure and acid sites.The PONA contents in gasoline product obtained from diesel cracking over the Y zeolite and the β zeolite were analyzed. The results are shown in Figure 2.

Figure 2 PONA content in gasoline product obtained from diesel cracking of Y zeolite and β zeolite—Y;—β
It can be seen from Figure 2 that the olefin content in gasoline obtained from diesel cracking over the β zeolite is higher than that over the Y zeolite, especially the contents of C5=, C6=, C7=and C8=olefins. J. S. Buchanan[3]has shown that C5=—C8=olefins are the precursors that can be cracked into C3=olefin on the ZSM-5 zeolite. So that the high C5=—C8=olefins selectivity achieved by the
β zeolite will be in favor of making propylene.
The above-mentioned results can be attributed to the difference in acidity and pore structure of these three zeolite samples.
The aged Y zeolite, aged β zeolite, and aged ZSM-5 zeolite and their mixed samples were tested in the VGO micro-reactor unit, with the results presented in Table 7. It can be seen from Table 7 that under the same inventory of zeolite samples, the C3—C4yield,the C3=yield, and the C3=selectivity achieved over the mixture of Y, β and ZSM-5 zeolites are higher than that of the mixture of Y and β zeolites or the mixture of Y and ZSM-5 zeolites. According to these data, it can be inferred that the β zeolite plays a ‘relay role’ between the Y zeolite and the ZSM-5 zeolite in the course of cracking reaction. The Y zeolite can crack the bigger hydrocarbon molecules to middle molecules, while the β zeolite can crack the middle molecule to C5=—C8=olefins, and after that the ZSM-5 zeolite can crack C5=—C8=olefins to propylene.
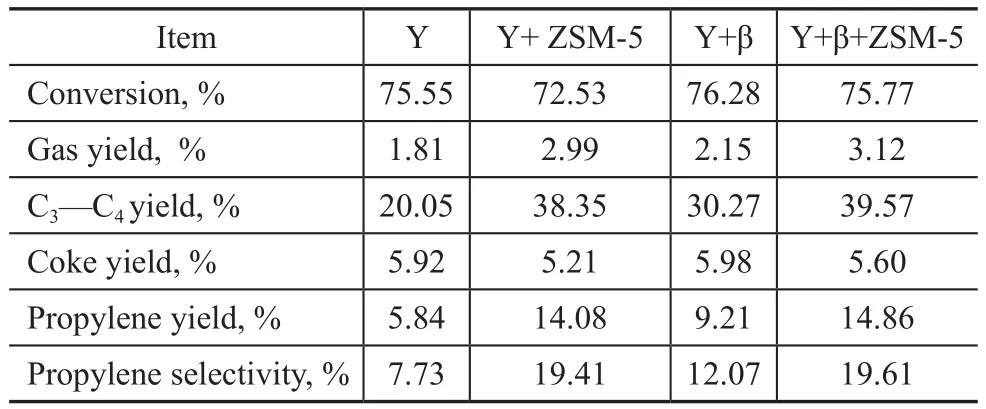
Table 7 Results of VGO cracking in micro-reactor unit over different zeolite samples(Reaction temp.: 500 °C, weight of feedstock: 1.7 g, regeneration temp.: 600 °C)
4 Conclusions
The Y zeolite, β zeolite and ZSM-5 zeolite have different cracking capability and distribution of products emanating from hydrocarbons of different molecular size. The β zeolite can produce much more C5=—C8=olefins, which are then cracked on ZSM-5 zeolite to propylene. There is a relay action among the Y, β, and ZSM-5 zeolites, so that the composite consisting of the Y, β, and ZSM-5 zeolites shows higher propylene yield and selectivity.
[1]Wang Chengqiang, Ouyang Ying, Luo Yibin. Ring opening performance of monocyclic naphthene over phosphorus modified beta zeolite[J]. Petroleum Processing and Petrochemicals, 2016, 47(8): 7-12(in Chinese)
[2]Qin Lihong, Li Haiyan, Li Jun, et al. Synthesis of nano-ZSM-5 in ultra-concentrated system and its performance in diesel hydrodewaxing[J]. China Petroleum Processing and Petrochemical Technology, 2016, 18(4): 25-31
[3]Buchanan J S, Santiestban J G, Haag W O. Mechanistic considerations in acid-catalyzed cracking of olefins[J]. J Catal, 1996, 158 (1): 279-287
- 中國煉油與石油化工的其它文章
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Emission-Reductive and Multi-Objective Coordinative Optimization of Binary Feed for Atmospheric and Vacuum Distillation Unit
- A pH Model for Calculating the pH Value of Mixed–Acid–Base Equilibria of Overhead Condensing Systems in Crude Distillation
- Simultaneous Biological Removal of p-Cresol, Sulfide and Nitrate by Denitrification
- The Effects of Ultrasonic Treatment on the Molecular Structure of Residual Oil
- Degradation of Nitrobenzene Wastewater via Iron/Carbon Micro-electrolysis Enhanced by Ultrasound Coupled with Hydrogen Peroxide

