Controlled release tablets of verapamil hydrochloride
Treephu Kimlek,Tossaporn Prasertporn,Jiramet Sawantranon, Chaisan Sriwichupong,Garnpimol Ritthidej
Pharmaceutical Technology Field,Department of Pharmaceutics and Industrial Pharmacy,Faculty of Pharmaceutical Sciences,Chulalongkorn University,Bangkok,Thailand
Controlled release tablets of verapamil hydrochloride
Treephu Kimlek*,Tossaporn Prasertporn,Jiramet Sawantranon, Chaisan Sriwichupong,Garnpimol Ritthidej
Pharmaceutical Technology Field,Department of Pharmaceutics and Industrial Pharmacy,Faculty of Pharmaceutical Sciences,Chulalongkorn University,Bangkok,Thailand
A R T I C L E I N F O
Article history:
Available online 24 November 2015
Verapamil
Controlled release
HPMC
Sodium alginate
Verapamil is a calcium channel blocker drug which reduces heart rhythm and blood pressure in high blood pressure patient. The steady state of this drug has to be continually controlled to obtain optimal effcacy.In addition,patient compliance needs to be considered.Therefore,controlled release tablet of verapamil hydrochloride was essentially developed using hydrophilic matrix polymer,namely hydroxypropylmethyl cellulose(HPMC)and sodium alginate,and compared to marketed product[1].HPMC,a pH-independent polymer,hydrates to form a gel layer at the surface of the tablet to sustain the drug release.Sodium alginate,a pH-dependent gelling polymer, converts to insoluble alginic acid after exposure to acidic condition in stomach.The development of verapamil hydrochloride controlled release tablet was to vary the amount of HPMC and sodium alginate.Alteration of the type and quantity of excipients were included.Tablets were prepared by wet granulation method.Physical tests were evaluated on tablet such as appearance,thickness,hardness and weight variation.In dissolution test,USP monograph was followed using USP apparatus II(paddle).UV spectrophotometer was used to measure the amount of drug release[2],and then f1,f2 values were calculated.In assay study,high-pressure liquid chromatography was performed.
The results showed that all formulations obviously passed the physical tests.The type and amount of polymer signifcantly affected the release of drug.For the effect of the variation in type and amount of rate controlling agents,change in amount of sodium alginate slightly affected the release of the drug while change in amount of HPMC had a great impact because the forming of gel layer at early stage(pH 1.2)created barrier to control the drug release whereas at buffer stage(pH 6.8),sodium alginate swelled and caused a synergistic control release effect (Fig.1a).For other excipients,decrease in the amount of microcrystalline cellulose,used as a disintegrant and magnesium stearate,used as a lubricant could reduce tablet disintegration.Finally,povidone K30 as a binder agent could increase granule compaction but with amount limitation.Formulation with accepted range of drug release in USP monograph was satisfactorily obtained and similar to the marketed product. This formulation contained 2.67%of HPMC and 11.33%of sodium alginate.The f1 and f2 values were 13.30 and 61.49,respectively(Fig.1b).
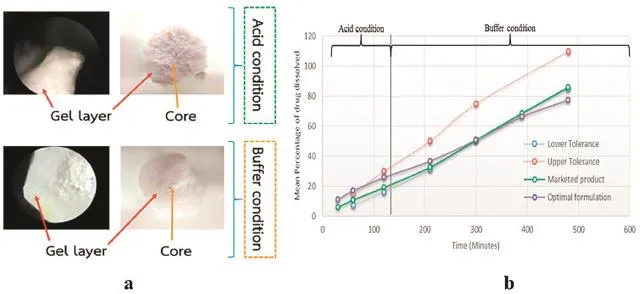
Fig.1–Gel layer forming of tablet in acid and buffer condition.(a)Dissolution profle of the selected formulations prepared with small scale and large scale production:HPMC:Sodium alginate=2.67:11.33 compared to marketed product(b).
Acknowledgement
The authors acknowledge the fnancial support received from Thailand Center of Excellence for Life Sciences(TCELS).
R E F E R E N C E S
[1]Abbott Laboratories.Product Monograph:ISOPTIN?SR [monograph on the Internet],
[2]The United States Pharmacopoeial Convention.USP36-NF31, vol.1.2013.p.5556–5563.
[3]Qiu Y,Zhang G.Development of modifed-release solid oral dosage forms.In:Developing solid oral dosage forms: pharmaceutical theory and practice.2009.p.501–510.
*E-mail address:tuarus_f@hotmail.com.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.027
1818-0876/?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
