Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
Nurdiana Nurdiana, Elly Mayangsari, Bayu Lestari, Bambang Setiawan
1Department of Pharmacology, Faculty of Medicine, University of Brawijaya, Malang, East Java, Indonesia
2Research Center for Toxicolgy, Cancer and Regenerative Medicine, Department of Medical Chemistry and Biochemistry, Faculty of Medicine, University of LambungMangkurat, Banjarmasin, South Kalimantan, Indonesia
Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
Nurdiana Nurdiana1*, Elly Mayangsari1, Bayu Lestari1, Bambang Setiawan2
1Department of Pharmacology, Faculty of Medicine, University of Brawijaya, Malang, East Java, Indonesia
2Research Center for Toxicolgy, Cancer and Regenerative Medicine, Department of Medical Chemistry and Biochemistry, Faculty of Medicine, University of LambungMangkurat, Banjarmasin, South Kalimantan, Indonesia
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Soybean extract
Reproductive hormones
Male rats
Testis
Spermatogenesis
Objective:To analyze the effect of administering soybean extracts during pregnancy and breastfeeding on hormonal disturbances and impaired spermatogenesis in male rats born to mothers receiving soybean extract.Methods:Twenty-eight male rat puppies were divided into four groups: the control group (no treatment) and the groups male rat puppies whose mothers have been given soy extract of various doses (68.88 μg/mL, 137.76 μg/mL and 275.52 μg/mL). Those male rats were born to female rats given soybean extracts during their pregnancy and lactation until the age of one month. Analysis of the levels of LH, FSH and testosterone was performed by the ELISA technique. Testicular spermatogenesis was analyzed by histopathology.Results:FSH levels were significantly lower for all three doses of soy extract than those of the control group (P<0.05). FSH levels increased significantly in the group treated with the third dose of soybean extract relative to those of the first and second doses (P<0.05). LH levels were significantly lower for all three doses of soy extract than those of the control group (P<0.05). Testosterone levels were significantly lower for the third dose of soybean extract relative to those of the control group (P<0.05). With regard to histology of seminiferous tubules, it was found that the higher the dose of extract the more severe the constraints to spermatogenesis would be.Conclusion:Administration of soybean extract since the intrauterine period, during lactation and at the age of two months to male rats will lead to hormonal changes and impaired spermatogenesis.
1. Introduction
Endocrine disruptor (ED) is an exogenous substance or mixture capable of altering the function of the endocrine system and has adverse effects on intact organisms and their progeny and subpopulations[1]. Chemical compounds classified as ED are very diverse, such as industrial chemicals, pharmaceutical compounds, cosmetic compounds and heavy metals[2-4]. These compounds can be found by naked eye in the environment and as contaminants in the food chain and exposure in the work environment[5].
Phytoestrogens abundantly found in soybeans and soybean products have properties similar to estrogen or act as an anti-estrogen so as to be considered beneficial. However, the findings regarding phytoestrogen benefits are indirect and inconsistent. Exposure to estrogen compounds, especially in certain periods of life, will lead to malignancies and some anomalies in the reproductive system[6]. Structurally, phytoestrogens are similar to endogenous estrogen and have an affinity for estrogen receptors. There are various types of phytoestrogens, including isoflavones, prenylated flavonoids and coumestants. Genistein and daidzein are the most common of those compounds[7]. Previous studies demonstrated that genistein given in the maternal diet would significantly lead to growth inhibition of male progeny when given during lactation[8].
Impaired spermatogenesis and male infertility are conditions occurring as a result of the action of ED compounds. ED compounds will disturb biosynthesis, metabolism and action of hormones[9,10].Isoflavones may downregulate mRNA expression of follicle-stimulating hormone receptors, inhibinα, INHβB, androgen-binding protein and transferrin in Sertoli cells[11].
To date, there has been controversy over the finding of the effects of soybeans exposure with regard to hormonal disturbances and impaired spermatogenesis in boys born to mothers consuming ED compounds.
Hence, the purpose of present study was to analyze hormonal disturbances and impaired spermatogenesis in male rats born to mothers receiving soybean extract in pregnancy and lactation.
2. Materials and methods
2.1. Laboratory animals
This study was an experimental one. It used male rats born to pregnant female rats that were given soybean extracts during pregnancy and lactation until the age of one month. One-month-old rats were separated from their mothers and given soybean extractusing a feeding tube until 2 months of age. The control group did not receive soybean extract during the period. Twenty-eight male rat puppies treated were randomly divided into four groups: the control group (no treatment) and the groups treated with various doses of soybean extract (68.88 μg/mL, 137.76 μg/mL and 275.52 μg/mL).
2.2. Soybean extraction
The soybean plant used in the present study was of Argomulyo variety. It was obtained from the Indonesian Legumes and Tuber Crops Research Institute Malang of East Java, Indonesia. Dried soybeans were crushed to obtain soybean powder. Soybean powder was macerated in 95% methanol. The procedures for maceration were in accordance with previous studies. Soybean extract was orally administered to pregnant female rats, which was continued until lactation and the puppies were one month of age.
2.3. Analysis of hormone levels
Analysis of the levels of LH, FSH and testosterone was performed by ELISA technique. Analytical procedures were according to the instructions on the kit.
2.4. Histopathology
The testicles were subjected to histopathological analysis. The analytical procedures were in accordance with previous studies. The quality of spermatogenesis was analyzed based on Jonsen’s criteria[12,13].
2.5. Ethics
The present study passed the review of the ethics committee of Medicine Faculty of Brawijaya University Malang, East Java, Indonesia.
2.6. Statistical analysis
All data are presented in mean±SD. Differences in levels among treatment groups were analyzed by ANOVA using SPSS 16.0 statistical package. A P-value<0.05 was considered statistically significant.
3. Results
Figure 1 shows FSH levels for different treatment groups. FSH levels were significantly lower for all three doses of soybean extract than those of the control group (P<0.05). FSH levels increased significantly for the group treated with the third dose of soybean extract relative to the first and second doses (P<0.05). There were no significant differences in FSH levels between the first and second doses (P>0.05).
LH levels for different treatment groups are presented in Figure 2. LH levels were significantly lower for all three doses of soy extract than those of the control group (P<0.05). There were no significant differences in LH levels among all three doses of soybean extract (P>0.05).
Figure 3 shows testosterone levels for different treatment groups. Testosterone levels were significantly lower for the third dose of soybean extract than those of the control group (P<0.05). There were no significant differences in testosterone levels between the control group and the first and second doses (P>0.05).
In the control group, there were extremely light spermatogenesis disorder, many advanced spermatids, and the disorganization of the epithelium. On the first-dose group, there were less than five spermatozoa in each tubule and a few advanced spermatids. In the second and third doses groups, there were advanced spermatozoa and spermatid, but only early spermatids were found. At the third dose group, there was only spermatocytes. Hematoxylin eosin staining, magnification of 400 times (Figure 4).deviation;a: P<0.05 compared with the control group;b: P<0.05 compared with SE1;c: P<0.05 compared with SE2. SE: soy extract.
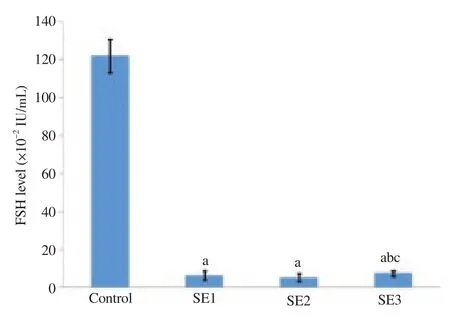
Figure 1. The levels of FSH in the control and treatment groups.
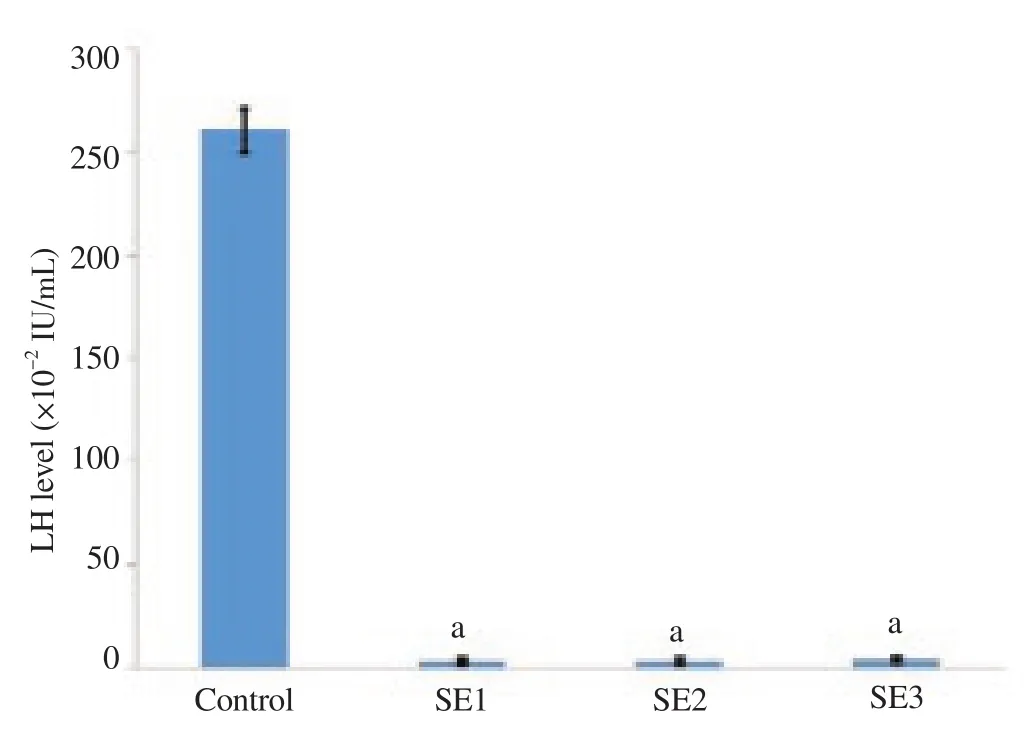
Figure 2. The levels of LH in the control and treatment groups.
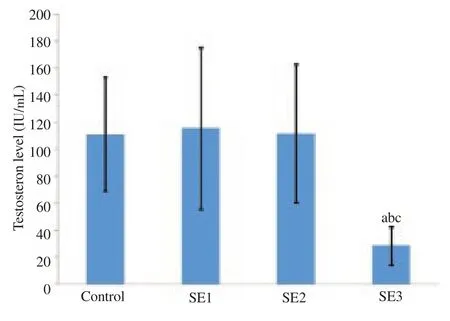
Figure 3. Testosterone levels in the control and treatment groups.
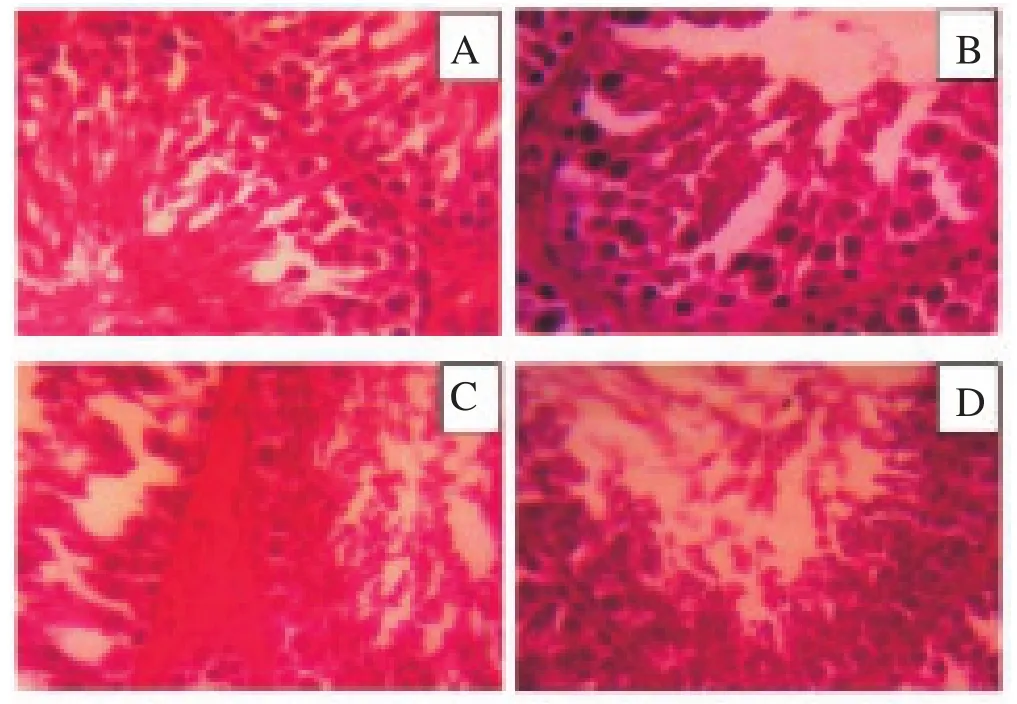
Figure 4. Histology of the seminiferous tubules of male rats in the control group (A) and groups treated with the first dose of soy extract (B), the second dose (C), and the third dose (D) (Magnification×400).
4. Discussion
In the present study, FSH levels decreased significantly for all three doses of soybean extract relative to the control group (P<0.05). With the third dose, there was a significant increase in FSH levels relative to the first and second doses (P<0.05). This indicates that administration of soybean extract will alter the bonding, release and metabolism of FSH. This is consistent with previous studies that ED compounds would impair endocrine function through altering the bonding, release and metabolism of endogenous hormones[14,15]. Fluctuating levels of FSH is underlain by the feedback mechanisms between the testis and hypothalamus. Decreased levels of FSH indicate hypothalamic impairment and increased levels of FSH indicate testicular impairment[16]. This is supported by histological findings of the present study. Meanwhile, the levels of LH were found to be significantly lower for the three groups of soybean extract treatment (P<0.05). With the third dose, there was a trend of increasing LH levels relative to the first and second doses, although no significant difference was found.
Interestingly, for testosterone, there was an increasing trend of levels for the group of the first and second dose treatment, although it was not different from the control group. These levels were drastically reduced for the third dose group of soybean extract administration. This indicates that, with the third dose, there was impaired testicular function. Previous studies demonstrated that genistein has a significant role in steroidogenesis in the adrenal glands and testes of rats and lowers testosterone levels. Meanwhile, the levels of FSH remain unchanged and the levels of LH tend to increase[17].
With regard to histology of seminiferous tubules, it was found that the higher the dose of extract the more severe the constraints to spermatogenesis would be. This finding is consistent with the previous ones that genistein delay spermatogenesis in male rats and humans[18,19].
In conclusion, administration of soybean extract since the intrauterine period, during lactation and at the age of two months to male rats will lead to hormonal changes and impaired spermatogenesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgements
We thank to all laboratory assistants at the Laboratory of Pharmacology of Medicine Faculty of Brawijaya University Malang of East Java, Indonesia. This study was funded by the grant of Educational Development Fund of Medicine Faculty of Brawijaya University Malang ofEast Java, Indonesia.
[1] Slama R, Bourguignon JP, Demeneix B, Ivell R, Panzica G, Kortenkamp A, et al. Scientific issues relevant to setting regulatory criteria to identify endocrine disrupting substances in the european union. Environ Health Perspect 2016; 124(10): 1497-1503.
[2] Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B 2009; 12: 206-223.
[3] De Coster S, van Larebeke N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J Environ Public Health 2012; 2012: 713696.
[4] Casals-Casas C, Desvergne B. Endocrine disruptors: From endocrine to metabolic disruption. Annu Rev Physiol 2011; 73: 135-162.
[5] Baccarelli A, Pesatori AC, Bertazzi PA. Occupational and environmental agents as endocrine disruptors: Experimental and human evidence. J Endocrinol Invest 2000; 23: 771-781.
[6] Bar-El DS, Reifen R. Soy as an endocrine disruptor: Cause for caution? J Pediatr Endocrinol Metab 2010; 23(9): 855-861.
[7] Khani B, Mehrabian F, Khalesi E, Eshraghi A. Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. J Res Med Sci 2011; 16(3): 297-302.
[8] Ball ER, Caniglia MK, Wilcox JL, Overton KA, Burr MJ, Wolfe BD, et al. Effects of genistein in the maternal diet on reproductive development and spatial learning in male rats. Horm Behav 2010; 57(3): 313-322.
[9] Andersson AM, J?rgensen N, Main KM, Toppari J, Rajpert-De Meyts E, Leffers H, et al. Adverse trends in male reproductive health: We may have reached a crucial ‘tipping point’. Intern J Androl 2008; 31: 74-80.
[10] Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. 2009 Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocrine Reviews 2009; 30: 293-342.
[11] Dejiao Y, Yanfeng Z, Linxi L, Hua X, Jie H, Yun L. Potential detrimental effect of soy isoflavones on testis sertoli cells. J Cent South Univ (Med Sci) 2014; 39(6): 598-604.
[12] Johnsen SG. Testicular biopsy score count - a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970; 1: 2-25.
[13] Ishihara M, Itoh M, Miyamoto K, Suna S, Takeuchi Y, Takekana I, et al. Spermatogenic disturbance induced by di (29ethylexyl) phthalate is significantly prevented by treatment with antioxidant vitamin in the rat. Intern J Androl 2000; 23: 85-94.
[14] Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: Is there a connection? Environ Health Perspect 2001; 109: 1175-1183.
[15] Colborn T, SaalFSv, Soto AM. Developmental effects of endocrinedisrupting chemicals in wildlife and humans. Environ Health Perspect 1993; 101: 378-384.
[16] Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 2008; 29: 988-997.
[17] Ohno S, Nakajima Y, Inoue K, Nakazawa H, Nakajin S. Genistein administration decreases serum corticosterone and testosterone levels in rats. Life Sci 2003; 74(6): 733-742.
[18] Delclos KB, Bucci TJ, Lomax LG, Latendresse JR, Warbritton A, Weis CC, et al. Effects of dietary genistein exposure during development on male and female CD (Sprague-Dawley) rats. Reprod Toxicol 2001; 15: 647-663.
[19] Goodin S, Shen F, Shih WJ. Clinical and biological activity of soy protein powder supplementation in healthy male volunteers.J Cancer Epidemiol Biomarkers Prev 2007; 16: 829-833.
ment heading
10.1016/j.apjr.2016.10.009
*Corresponding author: Nurdiana Nurdiana, Department of Pharmacology, Faculty of Medicine, University of Brawijaya, Malang, East Java, Indonesia.
Tel: +628123388428
E-mail: farmakodes@gmail.com
Foundation Project: This study was funded by the grant of Educational Development Fund of Medicine Faculty of Brawijaya University Malang ofEast Java, Indonesia.
 Asian Pacific Journal of Reproduction2016年6期
Asian Pacific Journal of Reproduction2016年6期
- Asian Pacific Journal of Reproduction的其它文章
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- Substitution of egg yolk with different concentrations of soybean lecithin in trisbased extender during bulls' semen preservability
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
