Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
Liu Chunshuang; Han Kang; Zhao Dongfeng; Guo Yadonag; Liu Lihong; Liu Fang; Zhao Chaocheng
(1. College of Chemical Engineering, China University of Petroleum, Qingdao 266580; 2. School of Earth Sciences, Northeast Petroleum University, Daqing 163318)
Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
Liu Chunshuang1; Han Kang1; Zhao Dongfeng1; Guo Yadonag1; Liu Lihong2; Liu Fang1; Zhao Chaocheng1
(1. College of Chemical Engineering, China University of Petroleum, Qingdao 266580; 2. School of Earth Sciences, Northeast Petroleum University, Daqing 163318)
The denitrifying sul fi de removal (DSR) process with bio-granules comprising both heterotrophic and autotrophic denitri fi ers can simultaneously convert nitrate, sul fi de and acetate species into di-nitrogen gas, elemental sulfur and carbon dioxide, respectively, at high loading rates. This study has determined that the reaction rate of sul fi de oxidized into sulfur could be enhanced in the presence of 1,2-naphthoquinone-4-sulphonate (NQS). The presence of NQS mitigated the inhibition effects of sul fi de species on denitri fi cation. Furthermore, the reaction rates of nitrate and acetate to nitrogen gas and CO2, respectively, were also promoted in the presence of NQS, thereby enhancing the performance of DSR granules. The advantages and disadvantages of applying the NQS-DSR process are discussed.
denitrifying sul fi de removal; NQS; inhibition; bio-granules
1 Introduction
Wastewater produced from oil industry, fertilizer industry and chemical industry usually contains high level of nitrogen and sulfur compounds. Nitrogen compounds contribute to eutrophication of water bodies, besides the risks associated with ammonia toxicity and offensive odor[1-2]. Reduced sulfur compounds, like sul fi de, are an environmental concern due to their toxicity, nasty smell and corrosive properties in the acidic environment[3-4]. Biological process can effectively remove nitrogenous compounds from wastewater[5-9]. Certain chemolithotrophic denitrifiers, such asThiobacillusdenitriticans, are capable of utilizing sulfide, thiosulfate or elemental sulfur (S0) as electron donors and nitrate or nitrite as electron acceptors to induce denitri fi cation reactions[10-13].
When both autotrophic and heterotrophic denitri fi ers were present in a reactor with unlimited nitrate species, Reyes-Avila, et al.[14]achieved simultaneous removal of nitrogen (NO3-→ N2), sul fi de (S2-→S0) and carbon (acetate→CO2); this process is referred to as the denitrifying sul fi de removal (DSR) process. Chen, et al.[15]cultivated biogranules comprising both autotrophic and heterotrophic denitrifiers to realize the enhanced DSR process performance, which was tantamount to 6.09 kgS/(m3·d) for sulfi de, 3.11 kgN/(m3·d) for nitrate, and 3.27 kgC/(m3·d) for acetate with an efficiency exceeding 93%. Liu,et al.[16]also successfully cultivated bio-granules while treating the high-salinity wastewater.
Controlling sulfide below an assessable level is crucial to maintaining the stability of a DSR reactor[17]. Aranda-Tamaura, et al.[18]argued that adding low levels of 1,2-naphthoquinone-4-sulphonate (NQS) can chemically oxidize S2-to S0/polysulphide, thereby signi fi cantly increasing the removal rate of S and N under autotrophic denitri fi cation conditions. Meza-Escalante, et al.[19]dosed a limited amount of NQS into a denitrifying reactor, thereby enhancing the removal of sul fi de and p-cresol. No study has utilized the NQS to enhance the DSR process for a high sulfide concentration. This study compares DSR performance under NQS presence and absence conditions in mixotrophic, heterotrophic and autotrophic mediums. Reaction pathways for DSR in the presence and absence of NQS conditions are discussed.
2 Experimental
2.1 Sludge
DSR granules were collected from a modi fi ed expanded granular sludge bed (EGSB) reactor for treating sul fi deand nitrate-laden saline wastewater[16]. The sludge was acclimated for 3 months in a lab-scale EGSB reactor under mixotrophic denitri fi cation condition without NaCl. The applied nitrate loading rate was 1.32 kgN/(m3·d) at a hydraulic residence time of 3.2 h. Na2S·9H2O and CH3COONa were used as electron donors at an S/N ratio of 1:1 and a C/N ratio of 1:1, respectively. The consortium showed high sulfide, nitrate and acetate removal efficiencies (>99%) in steady state with negligible accumulation of nitrite and sulfate species. Nitrous oxide was not detected in the biogas produced.
2.2 Batch assay
The batch assays for DSR granules were conducted in a 120-mL serum bottles containing 80 ml of media. Three media were used—a mixotrophic (S+N+C) medium containing 320 mg of S2-/L, 140 mg of NO3--N/L and 120 mg of acetate-C/L, an autotrophic (S+N) medium containing only 320 mg of S2-/L, 140 mg of NO3--N/L (without acetate), and a heterotrophic medium containing only 140 mg of NO3--N/L, 120 mg of acetate C/L (without sul fi de). The S/N and C/N molar ratios were maintained at 1:1 and 1:1, respectively. The composition of minerals and micronutrients was the same as that used by Chen, et al.[15]. The pH value of the medium was maintained at 7.5±0.2 with bicarbonate buffer. In order to evaluate the impact of NQS on DSR process, 100 μM NQS was supplemented. The sterilized serum bottles were fed with granules, fl ushed with helium gas for 10 min to expel oxygen gas, and then were sealed with a butyl rubber stopper. Abiotic controls in the absence of granular sludge were carried out to determine the chemical oxidation of acetate or/and sul fi de species by NQS.
The bottles were inoculated at 30±2 ℃. In each experimental condition, two serum bottles with identical feed were prepared to con fi rm the data reproducibility. Liquid samples from these bottles were collected regularly using a sterilized syringe.
2.3 Analytical methods
The concentrations of nitrate, nitrite, sulfate, and thiosulfate species in the collected liquor samples were measured by ion chromatography (ICS-3000; Dionex, USA) after being fi ltrated by 0.45-mm membrane fi lters. Sample separation and elution were performed using an IonPac AG4AAS4A-SC 4 mm analytical column with carbonate/ bicarbonate eluents (1.8 mmol/L of Na2CO3/1.7 mmol/L of NaHCO3at a rate of 1 cm3/min) and sulfuric regeneration (25 mmol/L of H2SO4at a rate of 5 cm3/min). The sul fi de concentration was determined using the methylene blue method[20]. The term ‘sul fi de’ in this study includes all species in liquid sample (H2S(aq), HS-and S2-). The acetate concentration and gas composition were determined by gas chromatography (6890; Agilent, USA). A pH/ oxidation-reduction potential (ORP) meter (type pH-25; Shanghai, China) determined the pH value and ORP of liquid samples. The amounts of suspended solids and VSS were measured according to the US Standard Methods[21]. Besides, the inoculated granular sludge was determined by 16S rRNA analysis. 16S rRNA gene fragments were PCR amplified using primers 338F (5’-ACT CCT ACG GGA GGC AGC AG-3’) and 806R (5’-GGA CTA CHV GGG TWT CTA AT-3’). The PCR ampli fi cation was performed according to the procedure referred to by Chen, et al[9]. The purified amplicon was quantified using a QuantiFluor-ST Fluorometer (Promega, USA), and then a composite sequencing library was constructed by combining equimolar ratios of amplicons from the sample. The resulting library for paired-end sequencing (2×250 bp) was analyzed on an Illumina Miseq platform at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). Raw sequence data of this study have been deposited to the NCBI Sequence Read Archive with accession No. SRS832283.
3 Results and Discussion
3.1 Microbial community of inoculated granular sludge
The phylogenetic classi fi cation of bacterial 16S rRNA sequence from the granular sludge sample after acclimation was illustrated based on genus (Figure 1). The identi fi ed dominant genera in the sludge wereThauera(39.2%),Arenimonas(9.3%),Thermomonas(22.4%),Aquimonas(4.03%),Rhodobacteraceae_unclassified (5.79%) andThiobacillus(2.34%) andCandidatus_Anammoximicrobium(12.9%), which were commonly found in denitrifying sul fi de removal process system.
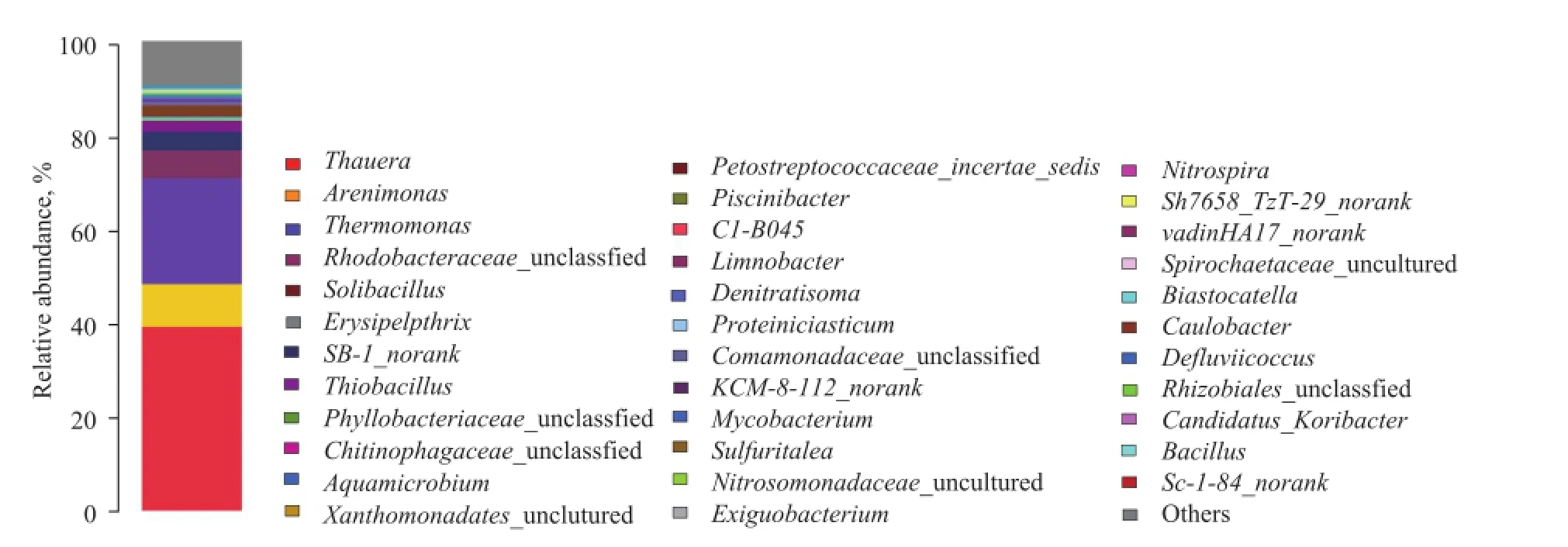
Figure 1 Taxonomic classi fi cation of the bacterial communities at genus level for inoculated granular.(Genus making up less than 0.5% of total composition in the sample was classi fi ed as “others”.)
3.2 Performance of DSR granules in the presence and absence of NQS
In the presence of NQS, the DSR granules in the mixotrophic (S+N+C) medium oxidized sul fi des faster than those in the autotrophic (S+N) medium, as shown in Figure 2a. For the mixotrophic medium, sulfides were completely removed in 10 h. For the autotrophic medium, the complete oxidation took place roughly in 14 h.
Conversely, within 10 h, the DSR granules oxidized 247 mg/L of sul fi des in the mixotrophic medium without NQS, a lower rate than that obtained in the presence of NQS. The control tests with mixotrophic and autotrohic media in the presence of NQS oxidized 188 mg/L of sul fi des, indicating to the contribution by non-biological sul fi de oxidation with NQS. Hence, in 10 h of testing, the DSR granules oxidized 237 mg/L (=247-10) of sulfides in the absence of NQS (the curve represented by the blue solid triangle), and oxidized 132 mg/L (=320-188) of sulfi des in the presence of NQS (the curve represented by the black solid square). During the last stage of oxidization in the absence of NQS, the DSR granules oxidized sulfi des much slower than during the fi rst 10 h. For instance, within 10—48 h, granules only oxidized another 35 mg/L (=70—35) of sul fi des (the curve represented by the blue solid triangle). Therefore, based on the time needed for complete oxidization of 400 mg/L of sulfides, the DSR granules performed much better in the presence of NQS. In all controls, the sulfate concentration remained at a low level, indicating that no sulfide was oxidized to sulfate via a non-biological pathway (Figure 2b). In the presence of NQS, the DSR granules did not oxidize sulfides to sulfate in the mixotrophic medium. However, in the autotrophic medium, the sulfate concentration reached roughly 100 mg/L in 24 h, and then gently increased afterwards to 115 mg/L. The same trend could be seen from the results obtained in the absence of NQS. No sulfate was accumulated in the mixotrophic medium. Conversely, 80 mg/L of sulfate were produced in 24 h in the autotrophic medium and increased to 106 mg/L in 48 h. This indicated that the presence of NQS did not change the formation of sulfate. In the presence of NQS, the DSR granules in the mixotrophic medium could remove all nitrates in 14 h (Figure 2c). Correspondingly, in the course of 14 h, in the autotrophic and heterotrophic media, the granules removed only 61.4 % (from 140 mg/L to 54 mg/L) of the nitrate and 66.4% (from 140 mg/L to 47mg/L) of the nitrate, respectively, indicating that the joint action of autotrophic denitri fi ers and heterotrophic denitri fi ers had a more important role in this granular system. In the absence of NQS, the granules removed only 66.4% of the nitrate (from 140 mg/L to 47mg/L) in the mixotrophic medium (Figure 2 c). The presence of NQS markedly enhanced the nitrate reduction by the DSR granules studied hereby.

Figure 2 Time courses of substances in DSR granules batch tests :(a) sul fi des; (b) sulfates;(c) nitrates; (d) nitrites; (e) acetates
Nitrites accumulated in all the tests except controls. The heterotrophic medium tests had shown obvious nitrite accumulation in the fi rst 6 h, reaching up to 85 mg/L and 63 mg/L, respectively, in the presence and absence of NQS, which might be ascribed to the inhibition of nitrate existing in the system on the removal of nitrite. The nitrite was then consumed and its concentration declined to zero after 48 h. The mixotrophic medium tests had modest nitrite accumulation in the fi rst 6 h, reaching up to 22 mg/L and 18 mg/L in the presence and absence of NQS, respectively. The accumulated nitrite was then consumed and its concentration declined to zero after 48 h. Conversely, the nitrite accumulated gradually in tests using the autotrophic media, and then leveled off at roughly 10 mg/L and 2 mg/ L, respectively, after 24 h in the presence and absence of NQS. In all the tests mentioned above, nitrite accumulation was lower in the presence of NQS. The presence of NQS could markedly reduce the nitrite accumulation by the granules studied hereby.
In the presence of NQS, the DSR granules removed 100% and 91.6% of acetate in the heterotrohic mediumand mixotrophic medium, respectively, within the first 14 h. Correspondingly, only 84.1% and 51.6% of acetate species were reduced in a 14 h test period in the absence of NQS in the heterotrohic medium and mixotrophic medium, respectively. (Figure 2 e.)
As a summary, Table 1 lists the species concentration measured experimentally and those calculated by mass balances before and after the tests.
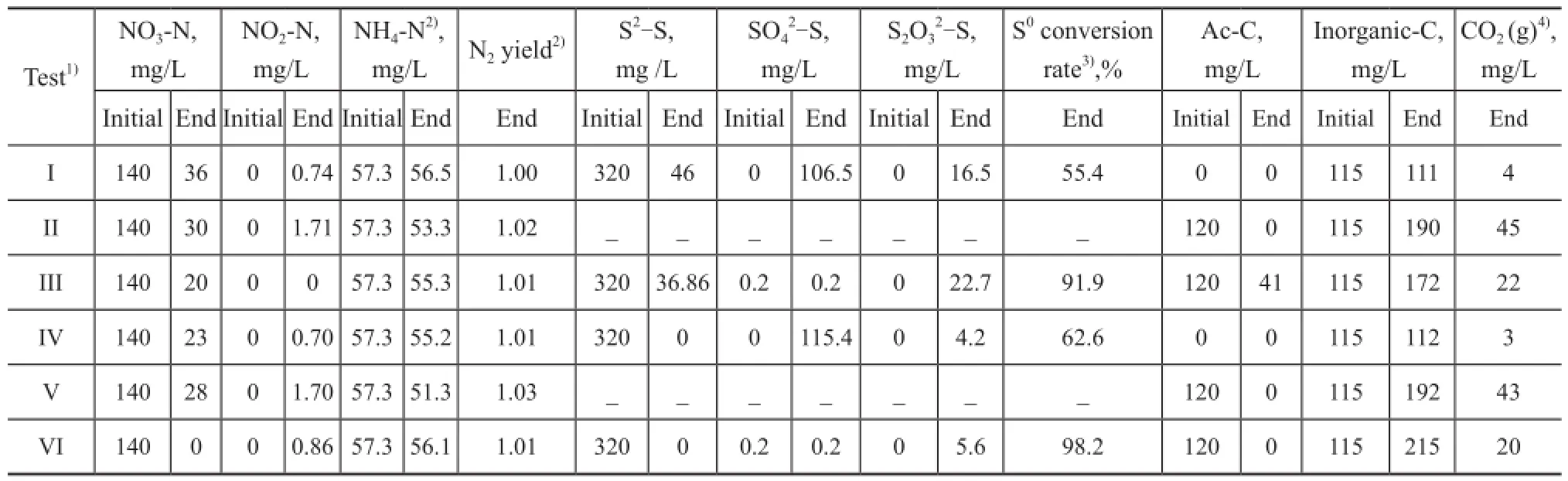
Table 1 Quantities of sulfur, nitrogen and carbon in batch tests
3.3 Metabolic pathway under NQS and non-NQS conditions
Reyes-Avila, et al.[14]proposed the following biochemical reactions for mixotrophic cultivation in the NQS-free environment:

Based on the quantity of sul fi des consumed in 48 h (Figure 2a) (the curve represented by black solid square), the corresponding concentration of NO2-N produced was 124 mg/L by Eq. (1). In the case in which all producedspecies were converted to N2via the heterotrophic denitri fi cation pathway (Eq. (2)), the consumed quantity of acetate-C species would be 79.8 mg/L. During the test, 82 mg/L of acetate was consumed (Figure 2e); therefore, the scheme proposed by Reyes-Avila, et al.[14]can be utilized to interpret the DSR data in this test by considering a minimal quantity of biomass growth (82-79.8=2.2mg/L). Since no sulfate was yielded in this test (Figure 2b), most oxidized sul fi des were converted to elemental sulfur.
In the presence of NQS, in addition to Eqs. (1) and (2) proposed by Reyes-Avila, et al.[14], the following reactions can occur in the mixotrophic medium.

In 10 h, 188 mg/L of sul fi de species were removed in the control tests in the mixotrophic and autotrohic media, and no sulfate was yielded in these tests, indicating that the elemental sulfur was yielded via the pathway in Eq. (3) in the presence of NQS. The removal of sul fi de and nitrate was enhanced in the autotrophic and mixotrophic media in the presence of NQS, indicating that a part of nitrate species were reduced via the pathway in Eq. (4) and the rate was faster than the pathway in Eq. (2). As well, the removal of acetate was increased in the heterotrophic and mixotrophic media in the presence of NQS, indicating that a part of acetate species were reduced via the pathway in Eq. (5) and the rate was faster than the pathway in Eq.(2).
3.4 Enhancing DSR process using NQS
The sul fi de concentration was important for maintaining the DSR performance. Chen, et al.[9]investigated the infl uence of high sul fi de loading on the performance of the DSR process in an EGSB reactor and determined that when the sul fi de concentration in the reactor exceeded 200 mg/L, the activity of heterotrophic denitri fi ers was inhibited markedly, leading to the breakdown of the DSR system.

Table 2 The reaction rates of sulfide, nitrate and acetate in autotrophic, heterotrophic, mixotrophic, autotrophic with NQS, heterotrophic with NQS and mixotrophic with NQS conditions. Batch tests were conducted
Reaction rates under different testing conditions and time periods are shown in Table 2. In the presence of NQS, the reaction rate of sul fi des to sulfur was promoted via Eqs. (1) and (3). With this rapid degradation of sul fi de species, the inhibition of denitrification by sulfide species was miti gated. Meanwhile, the reaction of nitrate and acetate species to form nitrogen gas and CO2, respectively, was promoted in the presence of NQS and the performance of DSR granules was enhanced. This reveals the main advantages of using the NQS-DSR process to treat wastewater containing high concentration of sul fi de and nitrate species. The disadvantages of applying the NQS include the easy loss of the dissolved NQS which can result in high operating cost and serious secondary pollution. An appropriate immobilization of NQS should be chosen to optimize the NQS-DSR performance with minimal loss of NQS.
The results of this study indicated that the presence of NQS could enhance the performance of DSR.
4 Conclusions
The DSR performance could be enhanced by the presence of NQS in both the mixotrophic and the autotrophic media. The reaction rate of sulfide oxidation into sulfur would be enhanced in the presence of NQS. The reaction rates of nitrate and acetate species to form nitrogen gas and CO2, respectively, were also promoted in the presence of NQS. The disadvantages of applying the NQS include the easy loss of the dissolved NQS, which can result in high operating cost and serious secondary pollution. An appropriate immobilization of NQS should be chosen to optimize the NQS-DSR performance with minimal loss of NQS.
Acknowledgements: The research was supported by the National Natural Science Foundation of China under Grant No. 21307160 and the Natural Science Foundation of Shandong Province under Grant No. ZR2013EEQ030.
[1] Liu M, Liu T, Peng Y, et al. Effect of salinity on N2O production during shortcut biological nitrogen removal from land fi ll leachate[J]. J Biosci Bioeng, 2014, 117 (5): 582-590
[2] Teixeira C, Magalhaes C, Joye S B, et al. The role of salinity in shaping dissolved inorganic nitrogen and N2O dynamics in estuarine sediment water interface[J]. Mar Pollut Bull, 2013, 66(1-2): 225-229
[3] Bahambhani Y, Singh M. Physiological effects of hydrogen sulfide inhalation during exercise in healthy men[J]. Appl Phys, 1990, 71(5): 1872-1877
[4] Deng L W, Tang Y, Wu Y. Mechanism of biological desulfurization and its progress[J]. Shanghai Environ Sci, 1998, 5(7): 35-39 (in Chinese)
[5] Xu X J, Chen C, Wang A J, et al. Simultaneous removal of sul fi de, nitrate and acetate under denitrifying sul fi de removal condition: Modeling and experimental validation[J]. J Hazard Mater, 2014(1/2), 264: 16-24
[6] Lee D J, Pan X, Wang A J, et al. Facultative autotrophic denitri fi ers in denitrifying sul fi de removal granules[J]. Bioresour Technol, 2013, 132: 356-360
[7] Guo H, Chen C, Lee D J, et al. Denitrifying sul fi de removalbyPseudomonassp. C27 at excess carbon supply[J]: Mechanisms. Bioresour Technol, 2015, 180: 381-385
[8] Huang C, Zhao Y, Li Z, et al. Enhanced elemental sulfur recovery with sequential sulfate-reducing, denitrifying sul fi de-oxidizing processes in a cylindrical-type anaerobic baf fl ed reactor[J]. Bioresour Technol, 2015, 192: 478-485
[9] Chen C, Wang A, Ren N, et al. Enhancing denitrifying sulfide removal with functional strains under micro-aerobic condition[J]. Process Biochem, 2010, 45(6): 1007-1010
[10] Xu X, Chen C, Lee D J, et al. Sulfate-reduction, sul fi deoxidation and elemental sulfur bioreduction process: modeling and experimental validation[J]. Bioresour Technol, 2013, 147, 202-211
[11] Yu H, Chen C, Zhang L, et al. Effect of dissolved oxygen on microbial community in simultaneous removal of carbon, nitrogen and sulfur process[J]. J Environ Sci-CHINA, 2013, 34(6): 304-310
[12] Wang A J, Du D Z, Ren N Q, et al. An innovative process of simultaneous desulfurization and denitrification by Thiobacillus denitrification[J]. J Environ Sci Health A, 2005, 40(10): 1939-1949
[13] Kleerebezem R, Meende R. Autotrophic denitrification for combined hydrogen sulfide removal from biogas and postdenitri fi cation[J]. Water Sci Technol, 2002, 45(10): 349-396
[14] Reyes-Avila J S, Razo-Flores E, Gomez J. Simultaneous biological removal of nitrogen, carbon and sulfur by denitri fi cation[J]. Water Res, 2004, 38(14/15): 3313-3321
[15] Chen C, Wang A J, Ren N Q, et al. Biological breakdown of denitrifying sulfide removal process in high-rate expanded granular bed reactor[J]. Appl Microbiol Biotechnol, 2008, 81(4): 765-770
[16] Liu C, Zhao C, Wang A, et al. Denitrifying sul fi de removal process on high-salinity wastewater[J]. Appl Microbiol Biotechnol, 2015, 99(15): 6463-6469
[17] Chen C, Wang A J, Ren N Q, et al. High-rate denitrifying sul fi de removal process in expanded granular sludge bed reactor[J]. Bioresour Technol, 2009, 100: 2316-2319
[18] Aranda-Tamaura C, Estrada-Alvarado M I, Texier A C, et al. Effects of different quinoid redox mediators on the removal of sulphide and nitrate via denitri fi cation[J]. Chemosphere, 2007, 69(11): 1722-1727
[19] Meza-Escalante E, Texier A-C, Cuervo-Lopez F, et al. Effects of different quinoid redox mediators on the simultaneous removal ofp-cresol and sulphide in a denitrifying process[J]. Water Sci Technol, 2009, 59(10): 1945-1950
[20] Greenberg A E, Clesceri L S, Eaton A D. Standard Methods for the Examination of Water and Wastewater[M]. 18th Ed. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC,1992
[21] APHA. Standard Methods for the Examination of Water and Wastewater[M]. 21sted. American Water Works Association and Water Environment Federation, Washington, DC, USA. 2005
Received date: 2015-09-17; Accepted date: 2015-11-30.
Dr. Liu Chunshuang, E-mail: liuchunshuang723@126.com
- 中國煉油與石油化工的其它文章
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Molecular Simulations of FCC Dry Gas Components Adsorption in Zeolite Y1
- Intrinsic Kinetic Modeling of Thermal Dimerization of C5Fraction
- Synthesis and Properties of Dendritic Long-Chain Esters as Crude Oil Flow Improver Additives
- Method for Control of Particle Size and Morphology of Paraf fi n/Polystyrene-Divinylbenzene Microcapsules
- Study on Absorption and Regeneration Performance of Novel Hybrid Solutions for CO2Capture

