Preparation of Mouse Embryonic Stem Cells and Cardiomyocyte Differentiation Induced with Retinoic Acid and Ascorbic Acid
Zhao Xun-wu, Zheng Peng, Huang Zhi-jun, Zeng Yue, Adegoke E O, and Zhang Gui-xue
College of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
Preparation of Mouse Embryonic Stem Cells and Cardiomyocyte Differentiation Induced with Retinoic Acid and Ascorbic Acid
Zhao Xun-wu, Zheng Peng, Huang Zhi-jun, Zeng Yue, Adegoke E O, and Zhang Gui-xue*
College of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
The experiment was designed to study effects of retinoic acid and ascorbic acid on differentiation of mouse embryonic stem cells to cardiomyocytes. Embryonic bodies (EB) were developed from mESC in suspension culture, different levels of concentration of retinoic acid and ascorbic acid were used to determine the optimal conditions for EB formation. The results showed that the optimal concentrations were 10-9mol · L-1and 0.1 mg · mL-1for retinoic acid and ascorbic acids, respectively. 50% of EB which was significantly (p<0.05) different from the control group developed to cardiomyocytes. In conclusion, retinoic acid and ascorbic acid had strong ability to promote cardiomyocyte differentiation of mouse embryonic stem cells. 10-9mol · L-1retinoic acid and 0.10 mg · mL-1ascorbic acids were recommended to induce differentiation of mouse ES cells toward cardiomyocytes.
embryonic stem cell, differentiation, retinoic acid, ascorbic acid
Introduction
Embryonic Stem (ES) cells can be gotten from inner cell mass of the early embryos or primordial germ cells. ES cells can differentiate into all three primary germ layers and form all the tissues and organs further. Some studies on differentiation of ES have been reported (Ren et al., 2008; R?nn et al., 2015; Chanda et al., 2013). Doetschman et al. (1985) find that ES cells can differentiate into beating cardiomyocytes spontaneously, which not only provides the developmental pattern of early cardiogenesis, but also ideal doner cell for cell therapy of damaged cardiomyocytes.
However, less than 3% to 5% of embryonic body (EB) formed by ES cells can differentiate into cardiomyocytes spontaneously. In this study, retinonic acid and ascorbic acid were taken to study mouse ES cellsderived cardiomyocytes in order to provide theoretical and practical basis for further researches on ES cell differentiation.
Materials and Methods
Experimental materials
KM mouses (Harbin Veterinary Research Institute), H-DMEM (Gibco), Fetal bovine serum (FBS, Hyclone), recombinant mouse leukemia inhibitory factor (LIF, Chemicon), β-mercaptoethanol (β-Me, Amresco), non-essential amino acid (NEAA, Sigma), gelatin (Sigma), all the trans-retinoic acids (RA, Sigma), ascorbic acid (Amresco), L-glutaminate (Amresco), trypsin (Amresco), AKP staining test kit (ZSGB-BIO), SABC immunocytochemical stain test kit (Boster), and DAB test kit (Boster).
Preparation of Mouse Embryonic Fibroblast (MEF) feeder layer
MEF feeder layer was produced by embryonic fibroblast that was obtained from 13.5 dpc mouse embryos. The method of preparation of MEF feeder layer was according to Zheng et al (2009).
Isolation and culture of ES cells
3.5 days blastula were cultured on MEF feeder layer with ES cell culture medium (H-DMEM+15% FBS+100 IU · mL-1penicillin+100 ug · mL-1streptomycin+0.1 mmol · mL-1β-Me+0.01 mmol · mL-1NEAA+0.01 mmol · mL-1L-glutaminate+1 000 IU · mL-1LIF) at 37℃ in 5% CO2and saturated humidity. When the column-shaped ICM appeared, ICM was picked out. Differentiated and trophoblastic cells surrounding ICM were removed and ICM was divided into 10 to 50 small clumps and then were cultured on MEF feeder layer. When the new colony appeared again, the colony was passaged with the same method and pure ES cells could be obtained after the colony passages. ES cells were identified by staining with alkaline phosphatase.
Induced differentiation of ES cells and identification of ES cell-derived cardiomyocytes
Undifferentiated ES cells, which came from the second to the forth passage, were cultured in the hanging drops of ES cells culture medium without LIF for 4 days to form EB and the culture method was referred to in the report by Chen et al (2011). EB were cultured in the suspension culture medium that contained dimethyl sulphoxide (DMSO) with retinoic acid (10-7mol · L-1, 10-8mol · L-1, and 10-9mol · L-1) and ascorbic acid (0.05 mg · mL-1, 0.10 mg · mL-1, and 0.15 mg · mL-1) for 3 to 5 days, then plated onto gelatincoated culture dishes containing the culture medium with retinoic acid and ascorbic acid for 5 to 7 days to differentiate into cardiomyocytes, and the culture medium was changed every 2 days. The cardiacspecific protein-desmin was identified with SABC immunocytochemical stain test kit and DAB test kit staining, primary antibody agonistic mouse demsin (Boster).
Statistical analysis
All the data obtained from this experiment were analyzed and compared for significance using spss 11.5 at p<0.05.
Results
Result of isolation and culture of ES cells
It took about 4 to 5 days for the blastocysts attached to MEF feeder layer to hatch and expand without any experimental interference. 2 days later, the inner cell mass began to develop into large cylinderlike structure colony, the color of the center of cell colony became dark compared to the periphery part, and finally ICM pushed away MEF feeder layer (Fig. 1) for 2 to 5 days after small ES cell clumped passage. The new formed ES cell colonies had similar characteristics to feeder-dependent EC cells. They were of "bird nest" shape, small and smooth (Fig. 2). ES cells showed AKP strong positive, and the fibroblasts were negative (Fig. 3).
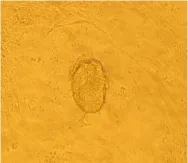
Fig. 1 Proliferative ICM, ×100
Differentiation of ES cell-derived cardiomyocytes
Undifferentiated ES cells were cultured in hanging drops of ES cell culture medium without LIF for 4 days to form EB. EB was composed of hundreds of cells, round, and its surface was rough with vecicles (Fig. 4).After the adherent culture, immunocytochemical stain of cardiac-specific protein-demsin appeared yellow which indicated that induced EB had expressed some cardiac-specific proteins (Fig. 5).

Fig. 2 ES cell colony, ×100
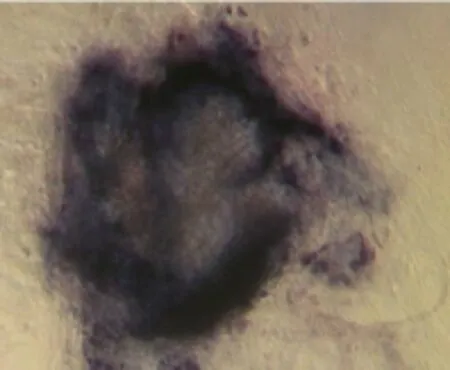
Fig. 3 Alkaline phosphatase staining of ES cell, ×100

Fig. 4 EB formed from ES cell, ×100

Fig. 5 EB of cardiac-specific protein identified by immunohistochemistry with anti-Demsin staining, ×200
The effects of different concentrations of retinoic acid and ascorbic acids on differentiation of ES cells are shown in Tables 1 and 2.
The optimal induced concentrations of retinoic and ascorbic acids were 10-8mol · L-1and 0.15 mg · mL-1, respectively.

Table 1 Effect of different concentrations of retinoic acids on differentiation of ES cells

Table 2 Effect of different concentrations of ascorbic acids on differentiation of ES cells
Discussion
Isolation and culture of ES cells
Some authors reported that the method of natural hatching (Evans and Kaufman, 1981) and immunosurgery (Martin, 1981) could get better effects on mouse blastocyst attachment. Though high quality ICM could be obtained by immunosurgery, the procedure of immunosurgery was quite complicated. It is unsuitable for the isolation and culture of ES cells because the immune reaction will damage ICM. Compared with immunosurgery, the method of natural hatching was simple. However, it was difficult to obtain pure ICM because there were many kinds of cells which would develop on MEF feeder layer including epithelioid-like cells, firoblast-like cells and trophoblastic cells.
The time of isolation was the key factor affecting the effect of isolation of ICM. The isolation effects of ICM was better when ICM developed into large cylinder-like structure. Enzymatic treatment and mechanical transfer were important for the expansion of ES cells, enzymatic treatment for longer time was harmful to most cells, and enzymatic treatment for a short time could not isolate the cells well to result in cell differentiation. This experiment contained both mechanical and enzymatic transfer methods for ES cell growth on feeder layers and obtained effects of ES cell isolation as other studies (Parnpai et al., 1998)
Culture medium with H-DMEM could promote the development of ES cells and provide energy for the growth of MEF feeder layer, which was helpful for blastocysts to attach to MEF feeder layer and to the propagation of ICM and the formation of ES colonies. β-mercaptoethanol promoted expansion and propagation of ES cells, and prevented the enzyme and protein in the cells from being oxidized and benefical to blastocysts in attaching to MEF feeder layer and forming the colonies of ES cells. β-mercaptoethanol deoxidized sulfur-containing compounds glutathione in the serum and promote the cells to propagate. The important biological function of LIF was that LIF inhibited the differentiation and apoptosis, and maintained the expansion and pluripotency of ES cells.
Differentiation of ES cell-derived cardiomyocytes
The culture process of ES cells differentiation included hanging drop culture, suspension culture and adherent culture. Undifferentiated ES cells were cultured in hanging drops for 2 to 4 days to form simple EB, EBs were cultured in culture medium adding inducer in order to form endoderm cell layer. The formation of endoderm cell was a pre-requisite to further differentiation. The effects of different concentrations of retinoic acid and ascorbic acid on differentiation of ES cells were studied. The induction efficiency of ascorbic acid was 50%, while that of retinoic acid was up to 60%. Retinoic acid and DMSO when used together could work efficiently, but DMSO was not suitable for clinical research because of it was cytotoxicity. When compared with DMSO, ascorbic acid was a bioactive and water-soluble vitamin, ascorbic acid didn't show cytotoxicity in a certain concentration range so that ES cell-derived cardiomyocytes could be used for clinical application. The main function of ascorbic acids was antioxidation, but some studies showed that antioxidation of ascorbic acid did not induce the differentiation of ES cells into cardiomyocytes (Takahashi et al., 2003). The mechanism of action of ascorbic acid in inducing ES cells to differentiate into cardiomyocytes need to be studied further. Other research reports could be referred to in order to further explore the differentiating mechanism and method of ESCs into cardiomyocytes (El-Sayed et al., 2014; Rabiee F et al., 2014) .
Conclusions
Retinoic acid and ascorbic acid had strong abilities to promote cardiomyocyte differentiation of mouse embryonic stem cells. 10-9mol · L-1retinoic acid and0.10 mg · mL-1ascorbic acid were recommended to induce the differentiation of mouse ES cells toward cardiomyocytes.
ARen X, Vincenz C, Kerppola T K. 2008. Changes in the distributions and dynamics of polycomb repressive complexes during embryonic stem cell differentiation. Molecular and Cell Biology,28(9): 2884-2895.
Chanda B, Ditadi A, Iscove N N, et al. 2013. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development cell. Cell,155(1): 215-227.
Chen M, Lin Y Q, Xie S L, et al. 2011. Enrichment of cardiac differentiation of mouse embryonic stem cells by optimizing the hanging drop method. Biotechnology Letters,4(33): 853-858.
Doetschman T C, H Eistetter, M Katz, et al. 1985. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, bood islands and myocardium. J Embryol Exp Morph,87: 27-45.
Evans M J, Kaufman M H. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature J,292(9): 154-156.
El-Sayed A K, Zhang Z T, Zhang L, et al. 2014. Pluripotent state induction in mouse embryonic fibroblast using mRNAs of reprogramming factors. Int J Mol Sci,15(12): 21840-21864.
Martin G R. 1981. Isolation of a pluripotent cell lines from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. J Proc Natl Acad Sci,78(2): 7634-7638.
Parnpai R, Minami W, Yamada M. 1998. A suitable passaging technique for the establishment of bovine embryonic stem like cell line. Therioginology,49(1): 241.
Rabiee F, Ghazvini F M, Zadegan F, et al. 2014. Induced expression of Fndc5 significantly increased cardiomyocyte differentiation rate of mouse embryonic stem cells. Gene,10(2): 127-137.
R?nn E, Guibentif C, Moraghebi R, et al. 2015. Retinoic acid regulates hematopoietic development from human pluripotent stem cells. Stem Cell Reports,4(2): 269-281.
Takahashi, Lord T B, Schulze P C, et al. 2003. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation J,107: 1912-1916.
Zheng P, Huang Z J, Lv Z H, et al. 2009. Study of several factors affecting on preparation of mouse embryonic stem cells. Life Science Journal,1(6): 1-4.
Q2
A
1006-8104(2015)-03-0062-05
Received 8 June 2015
Supported by the Scientific Research Foundation for Doctors of Northeast Agricultural University (2012RCB27); Open Projects of Key Laboratory of Animal Genetics, Breeding and Reproduction, College of Heilongjiang Province (GXZDSYS-2012-07)
Zhao Xun-wu (1988-), male, Master, engaged in the research of animal reproduction. E-mail: 806161051@qq.com
* Corresponding author. Zhang Gui-xue, professor, supervisor of Ph. D student, engaged in the research of animal reproduction. E-mail: gxzhang@ neau.edu.cn
 Journal of Northeast Agricultural University(English Edition)2015年3期
Journal of Northeast Agricultural University(English Edition)2015年3期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Design of Non-contact On-load Automatic Regulating Voltage Transformer
- Chinese Comprehensive Rural Reform: Institutional Vicissitude, Theoretic Framework and Content Structure
- Acceptability of Bush Meat as a Source of Animal Protein in Delta State, Nigeria: Implication for Extension Services
- Contents of Trace Metal Elements in Cow Milk Impacted by Different Feedstuffs
- Predatory Efficacy of Cotton Inhabiting Spiders on Bemisiatabaci, Amrascadevastans Thripstabaci and Helicoverpa armigera in Laboratory Conditions
- Effect of Bacillus subtilis and Pseudomonas fluorescens on Growth of Greenhouse Tomato and Rhizosphere Microbial Community
